Question
Methanoic acid is a monoprotic weak acid.
(a) The concentration of methanoic acid was found by titration with a 0.200mol \(dm^{−3}\) standard solution of sodium hydroxide, NaOH(aq), using an indicator to determine the end point.
Calculate the pH of the sodium hydroxide solution.
(b) Write an equation for the reaction of methanoic acid with sodium hydroxide.
(c) 22.5\(cm^3\) of NaOH(aq) neutralized 25.0\(cm^3\) of methanoic acid. Determine the concentration of the methanoic acid.
Answer/Explanation
Answer:
(a) «[\(OH^−\)] = 0.200 mol \(dm^{−3}\)»
ALTERNATIVE 1:
«pOH = −\(log_{10}\)(0.200) =» 0.699
«pH = 14.000 – 0.699 =» 13.301
ALTERNATIVE 2:
«[\(H^+\)] = \(\frac{1.00 \times 10^{-14}}{0.200}\) = » \(5.00 × 10^{−14}\) «mol \(dm^{−3}\)»
«pH = −\(log_{10}\)(\(5.00 x 10^{−14}\))» = 13.301
(b) HCOOH(aq) + NaOH(aq) → HCOONa(aq) + \(H_2O\)(l)
(c) «n(acid) = n(\(OH^−\))»
«[acid] = \(\frac{0.200 mol dm^{-3} \times 22.5 \times 10^{-3} dm^3}{25.0 \times 10^{-3} dm^3}\)» = 0.180 «mol \(dm^{−3}\)»
Question
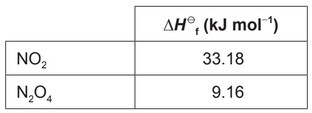
Nitrogen(IV) oxide, \(NO_2\), is a brown gas found in photochemical smog and has a pollutant causing acid deposition.
(a) Nitrogen(IV) oxide exists in equilibrium with dinitrogen tetroxide, \(N_2O_4\) (g), which is colourless.
\(2NO_2 (g) \rightleftharpoons N_2O_4 (g)\)
(i) At 100°C \(K_c\) for this reaction is 0.0665. Outline what this indicates about the extent of this reaction.
(ii) Calculate the value of Kc at 100°C for the equilibrium:
\(N_2O_4 (g) \rightleftharpoons 2NO_2 (g)\)
(iii) Calculate the standard enthalpy change, in kJ \(mol^{−1}\), for the reaction:
\(N_2O_4 (g)\) → \(2NO_2 (g)\)
(b) Deduce the Lewis structure of \(N_2O_4\).
(c) The NO bond lengths in \(N_2O_4\) are all \(1.19 × 10^{−10}\)m.
(i) Suggest what the bond lengths indicate about the structure of \(N_2O_4\).
(ii) Predict the ONN bond angle in \(N_2O_4\).
(d) Acid deposition is formed when nitrogen oxides dissolve in water. Write an equation for nitrogen(IV) oxide reacting with water to produce two acids.
Answer/Explanation
Answer:
(a) (i) reaction hardly proceeds
OR
reverse reaction/formation of \(NO_2\) is favoured
OR
«concentration of» reactants greater than «concentration of» products «at
equilibrium»
(ii) «\(K_c = \frac{1}{0.0665}\) =» 15.0
(iii) «\(\Delta H^{\theta}\) = 2(33.18) – 9.16 =» «+» 57.20 «kJ \(mol^{−1}\)»
(b) 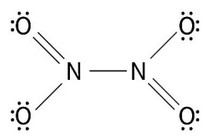
(c) (i) it has resonance structures
(ii) 110-\(120^o\)
(d) \(2NO_2(g) + H_2O(l) → HNO_2(aq) + HNO_3(aq)\)