Question
Which statements about the chlorine free radical are correct?
I. It has 18 electrons.
II. It is an uncharged species.
III. It is formed by homolytic fission.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Answer/Explanation
Markscheme
C
Examiners report
Question
Which of these reactions proceeds by a free radical mechanism in the presence of UV light?
A. C6H6 + Cl2 → C6H5Cl + HCl
B. C6H6 + 3H2 → C6H12
C. CH2CH2 + HBr → CH3CH2Br
D. CH3CH3 + Cl2 → CH3CH2Cl + HCl
Answer/Explanation
Markscheme
D
Examiners report
Question
Which is a propagation step in the free-radical substitution mechanism of ethane with chlorine?
A  → 2 •Cl
→ 2 •Cl
B •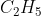 + Cl2 →
+ Cl2 → 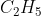 Cl + •Cl
Cl + •Cl
C •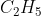 + •Cl →
+ •Cl → 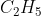 Cl
Cl
D 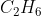 + •Cl →
+ •Cl → 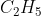 Cl + •H
Cl + •H
Answer/Explanation
Ans: B
Question
Which steps are involved in the free-radical mechanism of the bromination of ethane in the presence of ultraviolet radiation?
I. \({{\text{C}}_2}{{\text{H}}_6} + {\text{Br}} \bullet \to {{\text{C}}_2}{{\text{H}}_5} \bullet + {\text{HBr}}\)
II. \({{\text{C}}_2}{{\text{H}}_5} \bullet {\text{B}}{{\text{r}}_2} \to {{\text{C}}_2}{{\text{H}}_5}{\text{Br}} + {\text{Br}} \bullet \)
III. \({{\text{C}}_2}{{\text{H}}_5} \bullet + {\text{Br}} \bullet \to {{\text{C}}_2}{{\text{H}}_5}{\text{Br}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Answer/Explanation
Markscheme
D
Examiners report
Question
What is the mechanism for the reaction of propene with iodine in the dark?
A. electrophilic addition
B. electrophilic substitution
C. free radical substitution
D. nucleophilic substitution
Answer/Explanation
Markscheme
A