Question
Which is a propagation step in the free-radical substitution mechanism of ethane with chlorine?
A  → 2 •Cl
→ 2 •Cl
B •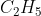 + Cl2 →
+ Cl2 → 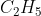 Cl + •Cl
Cl + •Cl
C •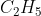 + •Cl →
+ •Cl → 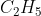 Cl
Cl
D 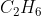 + •Cl →
+ •Cl → 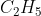 Cl + •H
Cl + •H
Answer/Explanation
Ans: B
Question
Which steps are involved in the free-radical mechanism of the bromination of ethane in the presence of ultraviolet radiation?
I. \({{\text{C}}_2}{{\text{H}}_6} + {\text{Br}} \bullet \to {{\text{C}}_2}{{\text{H}}_5} \bullet + {\text{HBr}}\)
II. \({{\text{C}}_2}{{\text{H}}_5} \bullet {\text{B}}{{\text{r}}_2} \to {{\text{C}}_2}{{\text{H}}_5}{\text{Br}} + {\text{Br}} \bullet \)
III. \({{\text{C}}_2}{{\text{H}}_5} \bullet + {\text{Br}} \bullet \to {{\text{C}}_2}{{\text{H}}_5}{\text{Br}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Answer/Explanation
Markscheme
D
Examiners report
Question
Which reaction occurs via a free-radical mechanism?
A. \({{\text{C}}_2}{{\text{H}}_6} + {\text{B}}{{\text{r}}_2} \to {{\text{C}}_2}{{\text{H}}_5}{\text{Br}} + {\text{HBr}}\)
B. \({{\text{C}}_2}{{\text{H}}_4} + {\text{B}}{{\text{r}}_2} \to {{\text{C}}_2}{{\text{H}}_4}{\text{B}}{{\text{r}}_2}\)
C. \({{\text{C}}_4}{{\text{H}}_9}{\text{I}} + {\text{O}}{{\text{H}}^ – } \to {{\text{C}}_4}{{\text{H}}_9}{\text{OH}} + {{\text{I}}^ – }\)
D. \({{\text{(C}}{{\text{H}}_3})_3}{\text{CI}} + {{\text{H}}_2}{\text{O}} \to {{\text{(C}}{{\text{H}}_3}{\text{)}}_3}{\text{COH}} + {\text{HI}}\)
Answer/Explanation
Markscheme
A