Question
What is the maximum number of electrons in energy level n = 4?
A. 8
B. 18
C. 32
D. 50
▶️Answer/Explanation
Markscheme: C
The maximum number of electrons that can occupy an energy level is given by the formula \(2n^2\), where \(n\) is the principal quantum number (the energy level).
For \(n = 4\):
\[2 \times (4)^2 = 2 \times 16 = 32\]
Therefore, the correct answer is C. 32.
Question
Which allotrope, oxygen or ozone, has the stronger bond between O atoms, and which absorbs higher frequency UV radiation in the atmosphere?
▶️Answer/Explanation
Markscheme: C
Since double bonds are stronger than single bonds, this means that the O-O bonds in oxygen are stronger than those in ozone.
The UV rays absorbed by oxygen atoms are very, very high in energy, but the Sun does not emit much of these rays. So oxygen atom absorption is not important.
The UV rays absorbed by oxygen molecules are high in energy and there is a medium amount of these rays emitted by the Sun. So oxygen molecule absorption is important, not only because UV rays are absorbed, but because the absorption helps create ozone.
The UV rays absorbed by ozone molecules are of medium energy and there is a lot of these rays emitted by the Sun. So ozone molecule absorption is very important.
Question
Which wavelength and energy of light will break bonds in ozone rather than oxygen molecules?
A. Shorter wavelength and lower energy
B. Shorter wavelength and higher energy
C. Longer wavelength and lower energy
D. Longer wavelength and higher energy
▶️Answer/Explanation
Markscheme: C
To break bonds in molecules, including ozone \(\left(\mathrm{O}_3\right)\), you need to provide energy that matches or exceeds the bond energy of the molecule. Bond energy is the energy required to break a chemical bond.
Ozone \(\left(\mathrm{O}_3\right)\) and oxygen \(\left(\mathrm{O}_2\right)\) molecules primarily absorb ultraviolet (UV) light. The ozone molecule has a higher bond energy than the oxygen molecule, and to break the bonds in ozone, you need to provide enough energy.
Now, let’s consider the wavelength and energy of light:
- Shorter wavelength corresponds to higher energy.
- Longer wavelength corresponds to lower energy.
This is because to break the bonds in ozone, you need lower-energy, longer-wavelength light compared to the higher-energy, shorter-wavelength light needed to break the bonds in oxygen.
Question
The table below shows the number of protons, neutrons and electrons present in five species.
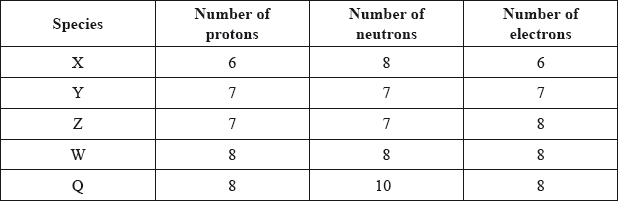
Which two species are isotopes of the same element?
A. X and W
B. Y and Z
C. Z and W
D. W and Q
Answer/Explanation
Markscheme
D
Examiners report
Question
Which quantities are the same for all atoms of chlorine?
I. Number of protons
II. Number of neutrons
III. Number of electrons
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Answer/Explanation
Markscheme
B