Question
What is the maximum number of electrons in energy level n = 4?
A. 8
B. 18
C. 32
D. 50
▶️Answer/Explanation
Markscheme: C
The maximum number of electrons that can occupy an energy level is given by the formula \(2n^2\), where \(n\) is the principal quantum number (the energy level).
For \(n = 4\):
\[2 \times (4)^2 = 2 \times 16 = 32\]
Therefore, the correct answer is C. 32.
Question
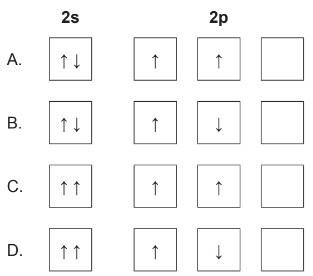
What is the correct ground state electron orbital configuration for \(2s^22p^2\)?
▶️Answer/Explanation
Markscheme: A
According to the Pauli Exclusion Principle, no two electrons in an atom can have the same set of four quantum numbers. This means that electrons in the same orbital must have opposite spins. Each orbital can accommodate a maximum of 2 electrons with opposite spins.
\(2 s^2\) represents the electron configuration for the 2s subshell, which can hold up to 2 electrons with opposite spin.
In the case of the \(2 p\) subshell, there are three individual orbitals labeled \(2 p_x, 2 p_y\), and \(2 p_z\). Each of these orbitals can hold up to 2 electrons with opposite spins. Two electrons are in the \(2 \mathrm{p}\) subshell, one in \(2 p_x\) with spin \(\uparrow\) and the other in \(2 p_y\) with spin \(\uparrow\).
To satisfy the Pauli Exclusion Principle, the two electrons in the \(2 p^2\) configuration must have opposite spins and must be placed in different orbitals within the \(2 p\) subshell.
So, the correct distribution is one electron in \(2 p_x\) with spin \(\uparrow\) and the other electron in \(2 p_y\) with spin \(\uparrow\). This ensures that both electrons have unique sets of quantum numbers, complying with the Pauli Exclusion Principle.
Question
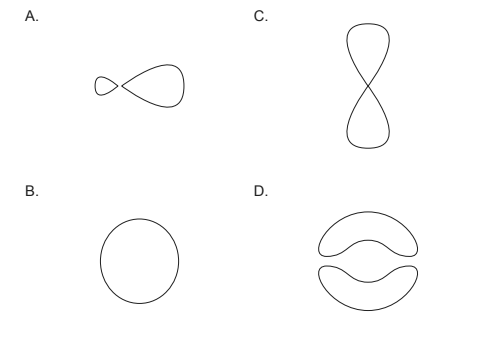
Which represents a p orbital?
Answer/Explanation
Ans: C
Question
Which shows the sub-levels in order of increasing energy in the fourth energy level of an atom?
A. \({\text{f}} < {\text{d}} < {\text{p}} < {\text{s}}\)
B. \({\text{p}} < {\text{d}} < {\text{f}} < {\text{s}}\)
C. \({\text{d}} < {\text{f}} < {\text{p}} < {\text{s}}\)
D. \({\text{s}} < {\text{p}} < {\text{d}} < {\text{f}}\)
Answer/Explanation
Markscheme
D
Question
Which species has the electron configuration of \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{8}}}\)?
A. Ni
B. \({\text{N}}{{\text{i}}^{2 + }}\)
C. Fe
D. \({\text{C}}{{\text{u}}^{2 + }}\)
Answer/Explanation
Markscheme
B