Question
Which information does the molecular formula provide?
A. The simplest ratio of atoms in a molecule
B. The actual numbers of atoms in a molecule
C. The number of molecules in one mole
D. The types of bonds in a molecule
▶️Answer/Explanation
Markscheme : B
A molecule is comprised of two or more atoms that have been chemically combined. A molecular formula is a chemical formula of a molecular compound that shows the kinds and numbers of atoms present in a molecule of the compound.
Question
A student heated a known mass of zinc powder in an open crucible until there was no further mass change and recorded the final mass.
What would the student be able to derive from this data?
I. Percentage composition of zinc oxide
II. Empirical formula of zinc oxide
III. Molecular formula of zinc oxide
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
▶️Answer/Explanation
Markscheme: A
The student would be able to derive the following information:
I. Percentage composition of zinc oxide – Yes, by comparing the initial and final masses, the student can determine the percentage of zinc in the zinc oxide formed.
II. Empirical formula of zinc oxide – Yes, by determining the moles of zinc and oxygen involved in the reaction, the student can find the empirical formula.
III. Molecular formula of zinc oxide – No, the molecular formula cannot be determined from this experiment alone. To find the molecular formula, additional information about the molar mass of the compound is needed.
Therefore, the correct answer is A. I and II only.
Question
A. \(1.0 \times 10^{-22}\)
B. \(2.0 \times 10^{-23}\) g
C. \(8.3 \times 10^{-24}\) g
D. \(1.2 \times 10^{-21}\) g
▶️Answer/Explanation
Markscheme: D
The molecular mass of a substance in grams is equal to its molar mass, which is numerically equal to the molecular weight (mass) of one mole of the substance.
The molecular formula for \(C_{60}\) (Buckminsterfullerene, also known as a buckyball) represents a molecule with 60 carbon atoms. The molar mass of \(C_{60}\) can be calculated by adding the atomic masses of 60 carbon atoms.
The atomic mass of carbon (\(C\)) is approximately 12.01 g/mol.
\[ \text{Molar mass of } C_{60} = 60 \times \text{Atomic mass of } C \]
\[ \text{Molar mass of } C_{60} = 60 \times 12.01 \, \text{g/mol} \]
\[ \text{Molar mass of } C_{60} = 720.6 \, \text{g/mol} \]
Now, to find the mass of one molecule of \(C_{60}\), we need to divide this molar mass by Avogadro’s number (\(N_A\)).
\[ \text{Mass of one molecule of } C_{60} = \frac{\text{Molar mass}}{N_A} \]
\[ \text{Mass of one molecule of } C_{60} = \frac{720.6 \, \text{g/mol}}{6.0 \times 10^{23} \, \text{mol}^{-1}} \]
\[ \text{Mass of one molecule of } C_{60} \approx 1.2 \times 10^{-21} \, \text{g} \]
Question
20\(cm^3\) of gas A reacts with 20\(cm^3\) of gas B to produce 10\(cm^3\) of gas \(A_xB_y\) and 10\(cm_3\) of excess gas A. What are the correct values for subscripts x and y in the empirical formula of the product \(A_xB_y\) (g)?
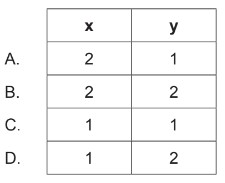
▶️Answer/Explanation
Markscheme: D
Let’s analyze the information provided:
1. \(20 \mathrm{~cm}^3\) of gas A reacts with \(20 \mathrm{~cm}^3\) of gas B.
2. The reaction produces \(10 \mathrm{~cm}^3\) of gas \(A_xB_y\) and \(10 \mathrm{~cm}^3\) of excess gas A.
From this information, we can deduce the following:
The initial moles of A and B are equal since the volumes are the same.
The reaction consumes all of B, as there is no excess of B mentioned.
\(10 \mathrm{~cm}^3\) of excess gas A remains unreacted.
Given that excess gas A is \(10 \mathrm{~cm}^3\), and \(10 \mathrm{~cm}^3\) of gas \(A_xB_y\) is produced, it implies that \(A_xB_y\) is formed by the combination of \(A\) and \(B\), and the remaining \(10 \mathrm{~cm}^3\) of A is in excess.
Since \(A_xB_y\) is formed from 20 \(\mathrm{cm}^3\) of A and 20 \(\mathrm{cm}^3\) of B, and the excess A is 10 \(\mathrm{cm}^3\), it suggests that the composition of \(A_xB_y\) is 1 part A and 2 parts B.
Therefore, the correct values for subscripts \(x\) and \(y\) in the empirical formula of the product \(A_xB_y\) are: \[ A_1B_2 \]
Question
Which observation would explain a systematic error for an experiment involving the combustion of magnesium to find the empirical formula of its oxide?
A. The crucible lid was slightly ajar during heating.
B. The product was a white powdery substance.
C. The crucible had black soot on the bottom after heating.
D. The flame colour during heating was yellow.
▶️Answer/Explanation
Markscheme: C
“The crucible had black soot on the bottom after heating,” would explain a systematic error in an experiment involving the combustion of magnesium to find the empirical formula of its oxide.
The combustion of magnesium involves reacting magnesium with oxygen to produce magnesium oxide (MgO). The presence of black soot indicates incomplete combustion. Incomplete combustion can result in the formation of carbon (soot) instead of complete conversion to magnesium oxide. This would lead to an underestimated mass of the magnesium oxide and, consequently, an inaccurate determination of the empirical formula.