Question
Double salts are substances with two cations and one anion. A hydrated sulfate containing two cations has this percentage composition.

(a) (i) Calculate the percentage of oxygen present in the double salt.
(ii) Determine the empirical formula of the double salt. Use section 6 of the data booklet.
(iii) The molar mass of the empirical formula is the same as the molar mass of the formula unit. Deduce the formula unit of the hydrated double salt.
(b) 1.20g of the double salt was dissolved in water and an excess of aqueous barium chloride was added, precipitating all the sulfate ions as barium sulfate.
(i) Formulate an ionic equation, including state symbols, for the reaction of barium ions with sulfate ions.
(ii) Calculate the mass of barium sulfate precipitate. Use your answer to part (a)(iii) and section 6 of the data booklet. (If you did not obtain an answer for part (a)(iii), use 400.0g \(mol^{-1}\) as \(M_r\) for the double salt, but this is not the correct value.)
Answer/Explanation
Answer:
(a) (i) «100-(7.09+5.11+16.22+14.91) =» 56.67 «%»
(ii) n(N): 7.09g/14.01g \(mol^{-1}\), n(H): 5.11g/1.01 g \(mol^{-1}\), n(S): 16.22g/32.07 g \(mol^{-1}\),
n(Co): 14.91g/58.93 g \(mol^{-1}\) and n(O): 56.67g/16.00 g \(mol^{-1}\)
OR
n(N): 0.506, n(H): 5.06, n(S): 0.506, n(Co): 0.253 and n(O): 3.54
0.506/0.253, 5.06/0.253, 0.506/0.253, 0.253/0.253, 3.54/0.253
OR
2.00, 20.0, 2.00, 1.00 14.00
\(N_2H_{20}S_2CoO_{14}\)
(iii) \((NH_4)_2Co(SO_4)_2·6H_2O\)
OR
\(Co(NH_4)_2(SO_4)_2·6H_2O\)
(b) (i) \(Ba^{2+}(aq) + SO_4^{2-}(aq) \rightleftharpoons BaSO_4(s)\)
(ii) «1.20g/395.29 g \(mol^{-1}\) salt = 2 x 3.04 x \(10^{-3}\) «mol» \(SO_4^{2-}\) =» 6.08 x x\(10^{-3}\) «mol»
«233.40 g \(mol^{-1}\) x 6.08 x\(10^{-3}\) =» 1.42«g»
OR
«(1.20g/400) x 2 g \(mol^{-1}\) =» 6.00 x \(10^{-3}\) «mol»
«233.40 g \(mol^{-1}\) x 6.00 x\(10^{-3}\) =» 1.40«g»
Question
An organic compound, X, with a molar mass of approximately \({\text{88 g}}\,{\text{mo}}{{\text{l}}^{ – 1}}\) contains 54.5% carbon, 36.3% oxygen and 9.2% hydrogen by mass.
(i) Distinguish between the terms empirical formula and molecular formula.
Empirical formula:
Molecular formula:
(ii) Determine the empirical formula of X.
(iii) Determine the molecular formula of X.
(iv) X is a straight-chain carboxylic acid. Draw its structural formula.
(v) Draw the structural formula of an isomer of X which is an ester.
(vi) The carboxylic acid contains two different carbon-oxygen bonds. Identify which bond is stronger and which bond is longer.
Stronger bond:
Longer bond:
(i) State and explain which of propan-1-ol, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\), and methoxyethane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\), is more volatile.
(ii) Propan-1-ol, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\), and hexan-l-ol, \({\text{C}}{{\text{H}}_{\text{3}}}{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{4}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\), are both alcohols. State and explain which compound is more soluble in water.
Graphite is used as a lubricant and is an electrical conductor. Diamond is hard and does not conduct electricity. Explain these statements in terms of the structure and bonding of these allotropes of carbon.
Graphite:
Diamond:
Answer/Explanation
Markscheme
(i) Empirical formula:
simplest (whole number) ratio of atoms/moles of each element present in a compound/molecule;
Molecular formula:
actual numbers of atoms/moles of each element present in a compound/molecule / whole number multiple of empirical formula;
(ii) \(n{\text{(C)}} = 4.54{\text{ (mol), }}n{\text{(H)}} = 9.11{\text{ (mol)}}\) and \(n{\text{(O)}} = 2.27{\text{ (mol)}}\);
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{O}}\);
Accept other valid method for calculation.
(iii) \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}\);
(iv) \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\);
Accept full or condensed structural formulas.
(v) \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_2}{\text{COOC}}{{\text{H}}_3}/{\text{C}}{{\text{H}}_3}{\text{COOC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}/{\text{HCOOC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}/{\text{HCOOCH(C}}{{\text{H}}_3}{{\text{)}}_2}\);
Accept full or condensed structural formulas.
(vi) Stronger bond:
C=O/double bond;
Longer bond:
C–O/single bond;
(i) methoxyethane/ \({\text{C}}{{\text{H}}_3}{\text{OC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\) as there are only dipole-dipole forces (and van der Waals’ forces) between molecules;
propan-1-ol has hydrogen bonding between molecules;
hydrogen bonding is stronger than dipole-dipole forces;
(ii) propan-1-ol/ \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{OH}}\) as it has a smaller hydrocarbon chain;
the longer (non-polar) carbon chain in hexan-1-ol decreases the attraction between the alcohol and the (polar) water molecules / OWTTE;
graphite:
forms flat hexagonal rings / layers of carbon atoms each (covalently) bonded to 3 other carbon atoms / trigonal planar around C / C has \({\text{s}}{{\text{p}}^{\text{2}}}\) hybridization;
layers are held together by weak intermolecular/van der Waals’ forces;
layers can slide over each other;
delocalization of electrons / free moving electrons;
diamond:
all carbon atoms are (covalently) bonded to 4 other carbon atoms / tetrahedral around C / C has \({\text{s}}{{\text{p}}^{\text{3}}}\) hybridization;
strong covalent bonds;
no delocalized electrons / OWTTE;
Examiners report
There were some vague and convoluted definitions in (a)(i) but thereafter the calculations were well done. Where difficulty was found, was in the formula of an ester in (v), (AS 10.1.11).
The answers to (b)(i) were reasonable, although it was common to state that the intermolecular bonding in methoxyethane is van der Waals‘. Some G2s took issue with the examination of ethers in organic chemistry; it was, in fact, examined under AS 4.3.2. In (ii), some mentioned a “larger molecule” rather than a “longer chain” and few were able to explain the attraction (or lack thereof) between the organic molecule and water.
Part (c) suggested that there is work to be done on understanding the structures of graphite and diamond. One particular mark lost was not to state that the reason diamond is hard is because the covalent bonds are strong.
Question
Smog is common in cities throughout the world. One component of smog is PAN (peroxyacylnitrate) which consists of 20.2% C, 11.4% N, 65.9% O and 2.50% H by mass. Determine the empirical formula of PAN, showing your working.
Answer/Explanation
Markscheme
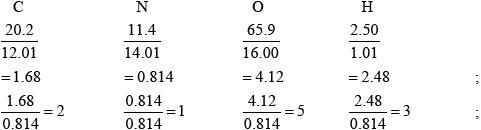
\({{\text{C}}_{\text{2}}}{\text{N}}{{\text{O}}_{\text{5}}}{{\text{H}}_{\text{3}}}\);
No penalty for use of 12, 1 and/or 14.
Award [1 max] if the empirical formula is correct, but no working shown.
Examiners report
It was pleasing to see the majority of candidates determine the correct empirical formula of PAN. Also, candidates showed the proper working with all the appropriate steps.