Question
What are the hybridizations of the atoms labelled 1, 2 and 3 in the molecule below?
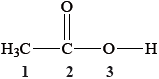
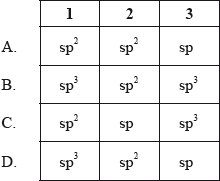
Answer/Explanation
Markscheme
B
Question
Which allotropes of carbon show \(s{p^2}\) hybridization?
I. Diamond
II. Graphite
III. \({C_{60}}\) fullerene
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Answer/Explanation
Markscheme
C
Question
Which molecules have \({\text{s}}{{\text{p}}^{\text{2}}}\) hybridization?
I. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\)
II. \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}\)
III. \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Answer/Explanation
Markscheme
B
Question
Which combination correctly describes the types of hybridization shown by the two carbon atoms labelled \(\alpha \) and \(\beta \) and the oxygen atom labelled \(\gamma \) in the molecule of paracetamol shown below?
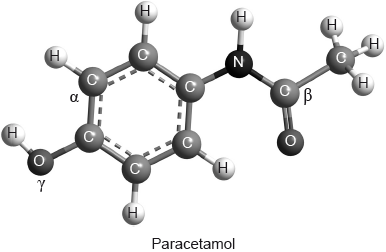
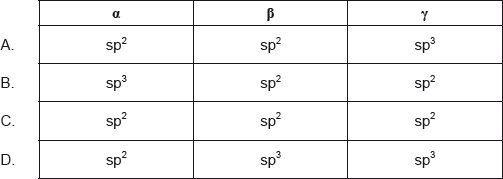
Answer/Explanation
Markscheme
A
Examiners report
Although delocalization in amide is not covered in the syllabus, answer C was also accepted here as there is significant double bond character in the nitrogen to carbon (of the carboxamide group) bond. The question will be amended before publication.
Question
Which combination describes the PH4+ ion?
Answer/Explanation
Markscheme
A