Question
What structural feature must a molecule have in order to undergo addition polymerization?
A. Two functional groups
B. A carbon–carbon double bond
C. Carbon atoms singly bonded together
D. A polar covalent bond
▶️Answer/Explanation
B
An addition polymer is a polymer formed by chain addition reactions between monomers that contain a double bond.
Question
Which reaction occurs via a free-radical mechanism?
A. \({{\text{C}}_2}{{\text{H}}_6} + {\text{B}}{{\text{r}}_2} \to {{\text{C}}_2}{{\text{H}}_5}{\text{Br}} + {\text{HBr}}\)
B. \({{\text{C}}_2}{{\text{H}}_4} + {\text{B}}{{\text{r}}_2} \to {{\text{C}}_2}{{\text{H}}_4}{\text{B}}{{\text{r}}_2}\)
C. \({{\text{C}}_4}{{\text{H}}_9}{\text{I}} + {\text{O}}{{\text{H}}^ – } \to {{\text{C}}_4}{{\text{H}}_9}{\text{OH}} + {{\text{I}}^ – }\)
D. \({{\text{(C}}{{\text{H}}_3})_3}{\text{CI}} + {{\text{H}}_2}{\text{O}} \to {{\text{(C}}{{\text{H}}_3}{\text{)}}_3}{\text{COH}} + {\text{HI}}\)
▶️Answer/Explanation
A
In A, This reaction is a substitution reaction, involving bromine free radicals, and this reaction can be initiated with either sunlight or heat.
Question
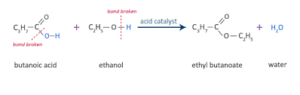
What is the name of the ester formed when \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\) react together?
A. Ethyl methanoate
B. Methyl ethanoate
C. Propyl methanoate
D. Methyl propionate
▶️Answer/Explanation
D
Methyl propionate can be prepared by esterification of propionic acid with methanol.
Question
What is the correct order of reaction types in the following sequence?
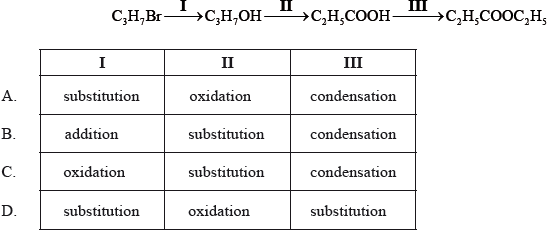
▶️Answer/Explanation
A
Reaction I is substitution reaction as Br is substituted by OH.
Reaction II is oxidation of alcohol where it is converted into carboxylic acid.
Reaction III is condensation of carboxylic acid to form an ester.
Question
Which reactants could be used to form the compound below?
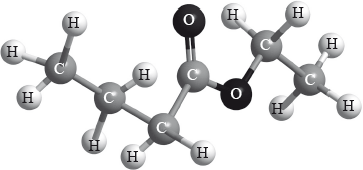
A. Butanoic acid and ethanol
B. Propanoic acid and ethanol
C. Ethanoic acid and propan-1-ol
D. Ethanoic acid and butan-1-ol
Answer/Explanation
A