Question
Stearic acid $\left(M_{\mathrm{r}}=284.47\right)$ and oleic acid $\left(M_{\mathrm{r}}=282.46\right)$ have the same number of carbon atoms. The structures of both lipids are shown in section 34 of the data booklet.
a. The iodine number is the number of grams of iodine which reacts with $100 \mathrm{~g}$ of fat. Calculate the iodine number of oleic acid.
[1]
b. The chemical change in stored fats causes rancidity characterized by an unpleasant smell or taste.
[2]
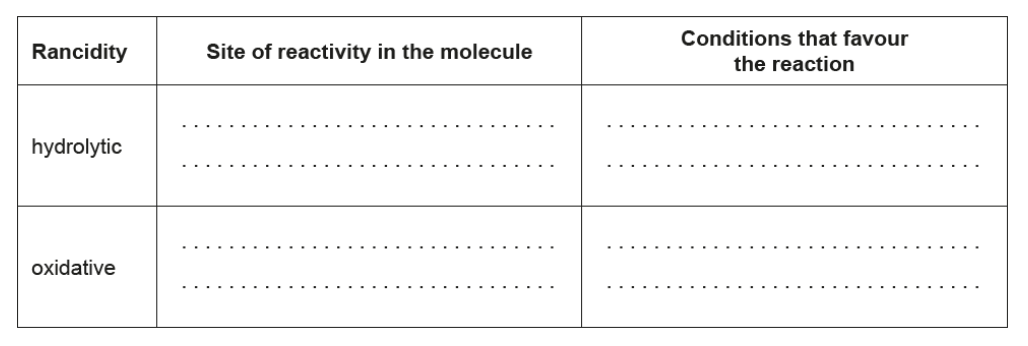
Compare hydrolytic and oxidative rancidity.
c. State one similarity and one difference in composition between phospholipids and triglycerides.
Similarity:
Difference:
▶️Answer/Explanation
Markscheme
a. «one $\mathrm{C}=\mathrm{C}$ bond»
«1 mole iodine : 1 mole oleic acid»
« $\frac{100 \times 253.80}{282.46}=» 89.85$ «g of $\mathrm{I}_2$ »
NOTE: Accept “90 «g of $\mathrm{I}_2 »$ “.
b.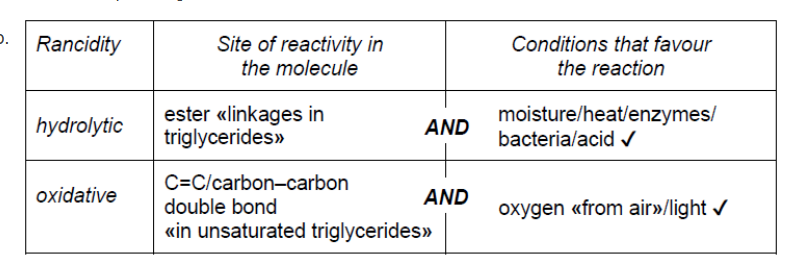
NOTE: Award [1] for any two sites or conditions from any of the four listed.
Accept “high temperature” for “heat”. Accept “lipase” for “enzyme”.
Do not accept just “double bond”.
Accept “air” for “oxygen” and “UV/sun” for “light”.
Ignore any reference to heathigh temperature as a condition for oxidative.
c. Similarity:
«derived from» propane-1,2,3-triol/glycerol/glycerin/glycerine
OR
«derived from» at least two fatty acids
OR
contains ester linkages
OR
long carbon chains
NOTE: Do not accept “two fatty acids as both a similarity and a difference”.
Do not accept just “hydrocarbon/carbon chains”.
Difference:
phospholipids contain two fatty acids «condensed onto glycerol» AND triglycerides three OR
phospholipids contain phosphate/phosphato «group»/residue of phosphoric acid $A N D$ triglycerides do not
NOTE: Accept “phospholipids contain phosphorus AND triglycerides do not”.
Accept “phospholipids are amphiphilic AND triglycerides are not” OR “phospholipids have hydrophobic tails and hydrophilic heads AND triglycerides do not”.
Question
Antimony oxide is widely used as a homogeneous catalyst for the reaction of benzene-1,4-dicarboxylic acid with ethane-1,2-diol in the production of polyethylene terephthalate (PETE).

\text { a. Deduce the repeating unit of the polymer and the other product of the reaction. }
b. State the class of polymer to which PETE belongs.
▶️Answer/Explanation
Markscheme
a. Repeating unit:
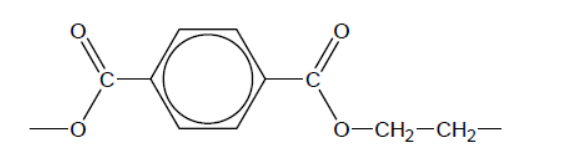
Other product: water/ $\mathrm{H}_2 \mathrm{O}$
Continuation bonds necessary for the mark.
Accept alternative repeating unit with $\mathrm{O}$ at other end.
Do not penalize square brackets or $n$.
[2 marks]
b. condensation
Accept polyester or thermoplastic.
[1 mark]
Question
Polymer nanocomposites often have better structural performance than conventional materials. Lithographic etching and metal coordination are two methods of assembling these nanocomposites.
Dendrimers are highly branched nanoparticles with a wide range of usage. One such dendrimer is PAMAM, or polyamidoamine.
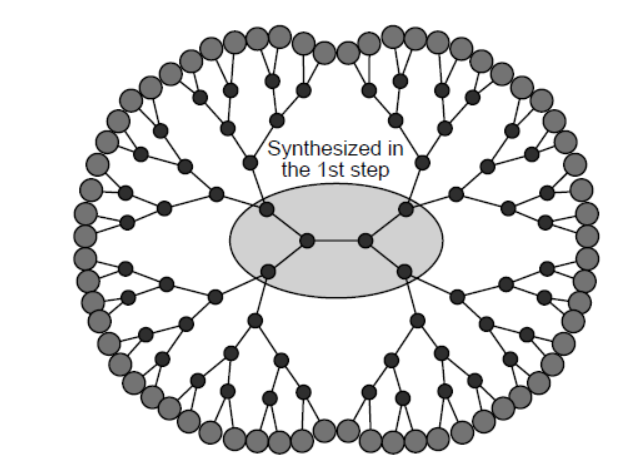
The first step in the synthesis is to make the core by reacting ethane-1,2-diamine with methylpropenoate.
c. Estimate the atom economy of this first step.
c.ii.Suggest, giving one reason, whether this is an addition or condensation reaction.
c.iiiSubsequent steps proceed under differing conditions, forming the dendrimer polymer with the following repeating unit.
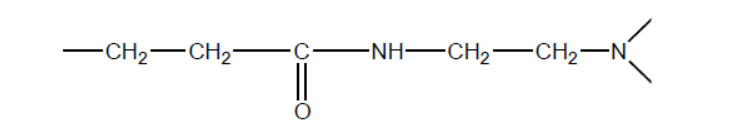
State the name of one functional group in this repeating unit.
▶️Answer/Explanation
Markscheme
c. $100 \%$
Accept “almost 100\%” if a catalyst is referred to.
[1 mark]
c.ii.addition $A N D$ no atoms removed/all atoms accounted for/no loss of water/ammonia/inorganic by-product/small molecules
OR
addition $\boldsymbol{A N D}$ there is only one «reaction» product
[1 mark]
c.iiiamido
OR
amino
Accept “amide/carboxamide/carbamoyl” for “amido”.
Accept “amine” for “amino”.
Accept “carbonyl”.