Question
When bromine water is shaken with a liquid organic compound, it is rapidly decolourised. What can be determined from this test?
A. The compound is an alcohol.
B. The compound is an alkane.
C. The compound is an alkene.
D. The compound is an iodoalkane.
▶️Answer/Explanation
C
Bromine water is an orange solution of bromine. It becomes colourless when it is shaken with an alkene. The presence of the C=C double bond allows alkene to react in ways that alkanes cannot. Alkenes can decolourise bromine water, but alkanes cannot.
The reaction between bromine and alkenes is an example of a type of reaction called an addition reaction. The bromine is decolourised because a colourless dibromo compound forms.
Question
Which conditions are required to obtain a good yield of a carboxylic acid when ethanol is oxidized using potassium dichromate(VI), \({{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}{\text{(aq)}}\)?
I. Add sulfuric acid
II. Heat the reaction mixture under reflux
III. Distil the product as the oxidizing agent is added
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
▶️Answer/Explanation
A
C2H5OH+K2Cr2O7/H+→CH3CHO→CH3COOH
Potassium dichromate is a very strong oxidizing agent. It acts very strongly in acidic medium hence adding sulfuric acid yields good amount of carboxylic acid.
The alcohol is heated under reflux with an excess of the oxidizing agent, to convert the aldehyde formed into carboxylic acid.
Ethanol is first oxidized to ethanal and then to ethanoic acid.
Question
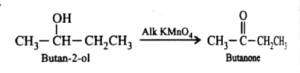
What is the product of the oxidation of butan-2-ol?
A. But-2-ene
B. Butanoic acid
C. Butanal
D. Butanone
▶️Answer/Explanation
D
Butan-2-ol is a secondary alcohol. The complete oxidation of secondary alcohols yields alkanones. The correct product is butanone.
Question
Which equations represent the incomplete combustion of methane?
I. \({\text{C}}{{\text{H}}_4}{\text{(g)}} + {\text{2}}{{\text{O}}_2}{\text{(g)}} \to {\text{C}}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(g)}}\)
II. \({\text{C}}{{\text{H}}_4}{\text{(g)}} + {\text{1}}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{CO(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(g)}}\)
III. \({\text{C}}{{\text{H}}_4}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{C(s)}} + {\text{2}}{{\text{H}}_2}{\text{O(g)}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
▶️Answer/Explanation
C
Only equation I represents complete oxidation of methane. This a process of heating a substance in the presence of sufficient air/O2.
The substance is completely oxidized to CO2 and H2O accompanied by evolution of a large amount of heat.
Reactions II and III are carried out in limited supply of O2, forming Carbon Monoxide and Coke respectively. These represent incomplete oxidation of methane.
Question
Which substance is not produced during the combustion of alkanes?
A. \({\text{C}}{{\text{O}}_{\text{2}}}\)
B. CO
C. C
D. \({{\text{H}}_{\text{2}}}\)
▶️Answer/Explanation
D
Complete combustion of alkanes produce \({\text{C}}{{\text{O}}_{\text{2}}}\).
Incomplete combustion of alkanes produce CO and C.
Combustion of alkanes does not produce \({{\text{H}}_{\text{2}}}\) .
