Question
Which structure has delocalized \(\pi \) electrons?
A. \({{\text{O}}_{\text{3}}}\)
B. CO
C. HCN
D. \({\text{C}}{{\text{O}}_{\text{2}}}\)
▶️Answer/Explanation
A
In ozone, there are 4 electrons delocalized in the pi system. Two occupy a molecular orbital containing node in the plane where the oxygen nuclei reside and the rest of the electron density is spread over the entire molecule above and below that plane. The other two occupy and orbital where there is a node in the plane of the nuclei and a node in a plane perpendicular to the plane of the nuclei to it right through central oxygen nucleus is located splitting the molecule in half. This is what spreads that negative charge to both the outer oxygens and why the resonance structure is drawn as: 
Question
How many bonding pairs and lone pairs of electrons surround the sulfur atom in the \({\text{S}}{{\text{F}}_{\text{4}}}\) molecule?
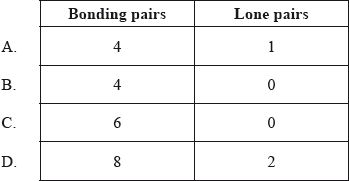
▶️Answer/Explanation
A
Around the core sulphur atom, SF4 contains five electron density zones (4 bonds and one lone pair). A trigonal bipyramid produces the see-saw structure. Sulphur Tetrafluoride contains 34 valence electrons, out of which it forms four covalent bonds and one lone pair of electrons on the core atom in its Lewis structure. On each fluorine atom, there are three lone pairs. It has the molecular geometry AX4E, and it creates a see-saw shape with a trigonal bipyramidal molecular geometry. SF4 is polar in nature and features sp3d hybridization.
Question
Which of the following best describes the formation of \(\pi \) bonds?
A. They are formed by the sideways overlap of parallel orbitals.
B. They are formed by the axial overlap of orbitals.
C. They are formed by the sideways overlap of an s and p orbital.
D. They are formed by the axial overlap of either s or p orbitals.
▶️Answer/Explanation
A
Pi bonds are formed by the sideways overlap of parallel orbitals along a direction perpendicular to the internuclear axis.
Sigma bond is formed by head-on positive (same phase) overlap of atomic orbitals along the internuclear axis.
Question
What is the hybridization of the carbon atom, and the number of \(\sigma \) and \(\pi \) bonds in the methanal molecule?
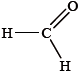
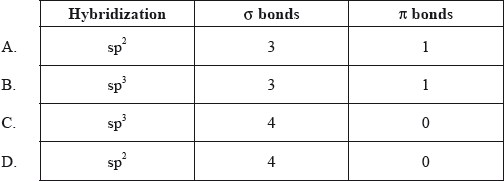
▶️Answer/Explanation
A
Here, double bonded carbon is sp2 hybridized. It is formaldehyde and it contains 3 sigma and 1 π bond so it’s hybridization will be sp2 and geometry will be trigonal planar.
Question
Which species have delocalized electrons?
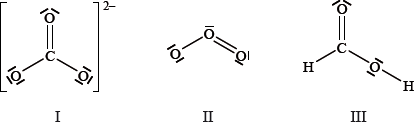
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
▶️Answer/Explanation
A
Carbonate and ozone have delocalized electrons as the O atoms here have both lone pairs and bond of electrons.
There are a total of 3 electron density regions or electron domains around the central C-atom in the HCOOH Lewis structure. All three electron domains are comprised of bond pairs; consequently, the central C-atom does not possess any lone pairs of electrons.
