Question
Draw diagrams to show how sigma (σ) and pi (π) bonds are formed between atoms. [2]
Sigma (σ):
Pi (π):
Answer/Explanation
Ans
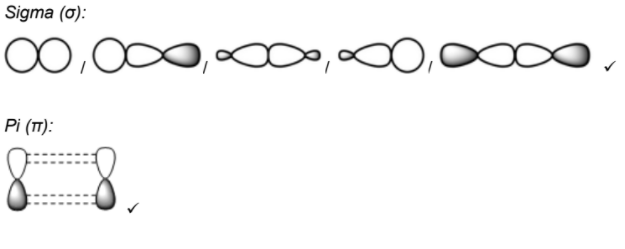
Accept any diagram showing end to end/direct overlap of atomic/hybridized orbitals and electron density concentrated between nuclei. Accept any diagram showing sideways overlap of unhybridized p/atomic orbitals and electron density above and below plane of bond axis.
Question
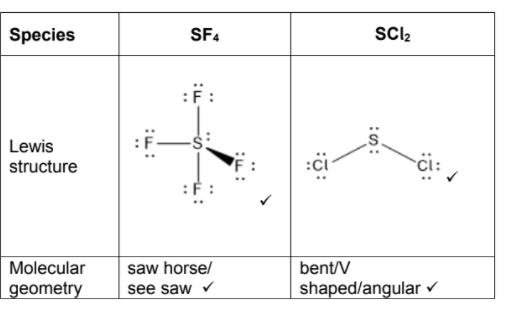
(a) Deduce the Lewis (electron dot) structure and molecular geometry of sulfur tetrafluoride, SF4, and sulfur dichloride,SCl2. [4]
Species | SF4 | SCl2 |
Lewis structure |
|
|
Molecular geometry | . . . . . . . . . . . . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
(b) Suggest, giving reasons, the relative volatilities of SCl2 and H2O. [3]
Answer/Explanation
Ans
a
b
H2O forms hydrogen bonding «while SCl2 does not»
SCl2 «much» stronger London/dispersion/«instantaneous» induced dipole- induced dipole forces
Alternative 1: H2O less volatile AND hydrogen bonding stronger «than dipole–dipole and dispersion forces»
Alternative 2: SCl2 less volatile AND effect of dispersion forces «could be» greater than hydrogen bonding
Question
Oxygen exists as two allotropes, diatomic oxygen, O2, and ozone, O3.
(a) (i) Draw a Lewis (electron dot) structure for ozone. [1]
(ii) Discuss the relative length of the two O–O bonds in ozone. [2]
(b)Explain why there are frequencies of UV light that will dissociate O3 but not O2. [2]
(c) Explain, using equations, how the presence of CCl2F2 results in a chain reaction that decreases the concentration of ozone in the stratosphere. [2]
Answer/Explanation
Ans:
7. a i 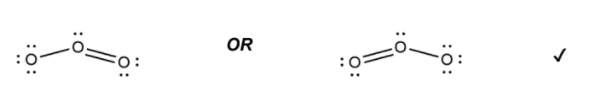
7. a ii
both equal delocalization/resonance
Accept bond length between 121 and 148 pm/ that of single O-O bond and double O=O bond for M1.
7. b
bond in O3 is weaker
OR O3 bond order 1.5/< 2
lower frequency/longer wavelength «UV light» has enough energy to break the O–O bond in O3 «but not that in O2» Do not accept bond in O3 is longer for M1. Accept “lower frequency/longer wavelength «UV light» has lower energy”.
7. c
CCl2F2(g) → •CClF2(g) + Cl•(g) ✔
Cl•(g) + O3(g) → O2(g) + ClO•(g)
AND
ClO•(g) + O3(g) → 2O2(g) + Cl•(g)
Do not penalize missing radical.
Accept:for M2: Cl•(g) + O3(g) → O2(g) + ClO•(g)AND
ClO•(g) + O(g) → O2(g) + Cl•(g)
