Question
Identify the hybridization of carbon atoms in this molecule
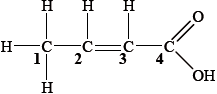
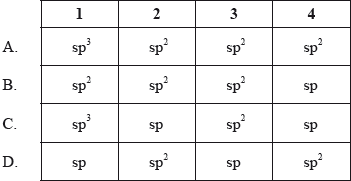
▶️Answer/Explanation
A
Hybridization of carbon in any organic molecule can be easily determined.
Carbon with single bond has sp3, carbon with double bond has sp2 hybridization, and carbon with triple bond has sp hybridization.
C1 has 4 single bonds, it has sp3 hybridization. C2, C3 and C4 have 1 double bond and 2 single bonds, hence they have sp2 hybridization.
Question
What is the hybridization of the carbon atom, and the number of \(\sigma \) and \(\pi \) bonds in the methanal molecule?
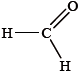
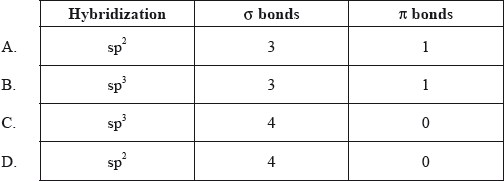
▶️Answer/Explanation
A
Here, carbon is double bonded, hence it is sp2 hybridized. It is formaldehyde and it contains 3 sigma and 1 π bond so it’s hybridization will be sp2 and geometry will be trigonal planar.
Question
What is the type of hybridization of the silicon and oxygen atoms in silicon dioxide?
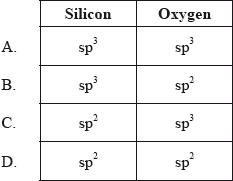
▶️Answer/Explanation
A
Silicon dioxide is a covalent, three-dimensional network solid in which each silicon atom is bonded to four oxygen atoms which are arranged tetrahedrally around it and each oxygen atom is attached to two silicon atoms by covalent bonds. Each corner is further shared by another tetrahedron. The entire crystal may thus be considered as a giant molecule in which eight-membered rings are formed with alternate silicon and oxygen atoms. SiO2 is a giant covalent molecule in which each silicon is surrounded by four oxygen atoms in a tetrahedral fashion and hence Si is sp3 hybridized. Oxygen is also sp3 hybridized.
Question
In which compound are all the carbon atoms \({\text{s}}{{\text{p}}^{\text{2}}}\)hybridized?
A. 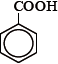
B. 
C. \({\text{C}}{{\text{H}}_{\text{2}}}{\text{CHC}}{{\text{H}}_{\text{3}}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHCHC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
▶️Answer/Explanation
A
In the first compound, all C atoms have on double bond. Carbon in COOH forms C=O and all other carbons in the benzene ring have C=C bond. Hence, it has all carbons with sp2 hybridization.
Question
Which statements about hybridization are correct?
I. The hybridization of carbon in diamond is \({\text{s}}{{\text{p}}^{\text{3}}}\).
II. The hybridization of carbon in graphite is \({\text{s}}{{\text{p}}^{\text{2}}}\).
III. The hybridization of carbon in \({{\text{C}}_{{\text{60}}}}\) fullerene is \({\text{s}}{{\text{p}}^{\text{3}}}\).
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
▶️Answer/Explanation
A
In diamond, each carbon atom is linked to four other carbon atoms. Thus, each carbon atom forms four bonds. The carbon atoms in diamond have sp3hybridization.
Graphite has layer structure in which several layers are formed. In graphite, each carbon atom is linked to three carbon atoms present in the same layer via strong covalent bonds. There are some interactions between two carbon atoms present in two adjacent layers. The carbon atom in graphite is sp2 hybridized.
Fullerenes have closed cage structure like a soccer ball or football. The hybridization of carbon in \({{\text{C}}_{{\text{60}}}}\) fullerene is \({\text{s}}{{\text{p}}^{\text{2}}}\).
