Question
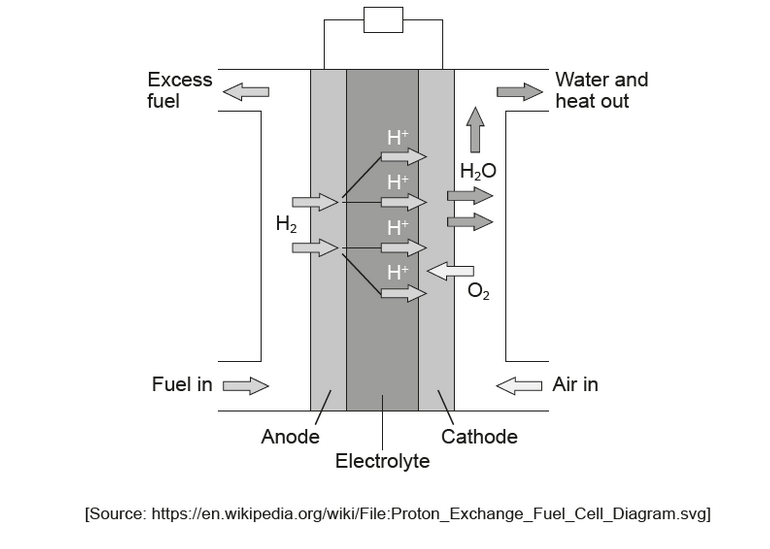
\text { A proton-exchange membrane (PEM) fuel cell uses pure hydrogen gas as the fuel and a proton exchange membrane as the electrolyte. }
A dye-sensitized solar cell (DSSC) uses light energy to produce electricity.
a. Deduce the half-equations for the reactions occurring at the electrodes.
Anode (negative electrode):
Cathode (positive electrode):
$\mathrm{b}(\mathrm{i})$ Calculate the cell potential, $E^\theta$, in $\mathrm{V}$, using section 24 of the data booklet.
b(ii)Suggest how PEM fuel cells can be used to produce a larger voltage than that calculated in (b)(i).
c. Suggest an advantage of the PEM fuel cell over the lead-acid battery for use in cars.
d(i)Outline the functions of the dye, $\mathrm{TiO}_2$ and the electrolyte in the operation of the DSSC.
Dye:
$\mathrm{TiO}_2$ :
Electrolyte:
d(iißSuggest an advantage of the DSSC over silicon-based photovoltaic cells.
▶️Answer/Explanation
Markscheme
a. Anode (negative electrode):
$$
\mathrm{H}_2(\mathrm{~g}) \rightarrow 2 \mathrm{H}^{+}(\mathrm{aq})+2 \mathrm{e}^{-}
$$
Cathode (positive electrode):
$$
\mathrm{O}_2(\mathrm{~g})+4 \mathrm{H}^{+}(\mathrm{aq})+4 \mathrm{e}^{-} \rightarrow 2 \mathrm{H}_2 \mathrm{O}
$$
(I)
NOTE: Accept any correct integer or fractional coefficients. Award [1 max] for M1 and M2 if correct half-equations are given at the wrong electrodes OR if incorrect reversed half-equations are given at the correct electrodes.
$$
\mathrm{b}(\mathrm{i})(+) 1.23 \text { « } »
$$
NOTE: Do not accept “-1.23 «V»”.
b(iiconnect several fuel cells in series
OR
increase pressure/concentration of reactant/hydrogen/oxygen
NOTE: Do not accept changes in $\left[\mathrm{H}^{+}\right] / \mathrm{pH}$ as they do not affect cell potential in this case.
Do not accept reference to quantity for “concentration”.
c. liquid in cell is less/not corrosive
OR
does not contain lead/toxic chemicals
OR
larger energy density/charge capacity/current per unit mass
OR
does not have to be charged prior to use / is always ready for use «as long as fuel is available»
d(i)Dye:
absorbs photons/light
OR
releases electrons
TiO2:
conducts current/electricity
OR
semiconductor
Electrolyte:
reduces/regenerates «the oxidized» dye
$\mathrm{d}$ (ii)Any one of:
cheaper/ease of manufacture
OR
plentiful and renewable resources «to construct DSSC cells»
use light of lower energy/lower frequency/longer wavelength
OR
use of nanoparticles provides large surface area for exposure to sunlight/sun/light
OR
can absorb better under cloudy conditions
operate at lower «internal» temperatures
OR
better at radiating heat away «since constructed with thin front layer of conductive plastic compared to glass box in photovoltaic cells»
better conductivity
more flexible/durable
NOTE: Accept “lower mass/lighter “so greater flexibility to integrate into windows etc.»” OR “greater power-conversion efficiency “with latest DSSC models””.