Question
How many protons, neutrons and electrons are present in each atom of \(^{{\text{31}}}{\text{P}}\)?
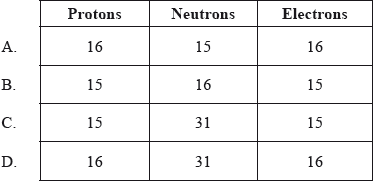
▶️Answer/Explanation
B
From the periodic table, we can see that the atomic number of phosphorus is 15, so that means that phosphorus has 15 protons.
An atom does not have a charge, so number of protons=number of electrons. Phosphorus also has 15 electrons.
Here, the mass number of phosphorus is given, which is 31. As mass number is equal to protons + neutrons in an atom, so mass number – protons = neutrons. 31 – 15 = 16, so phosphorus has 16 neutrons.
Hence, B is the correct answer.
Question
What is the atomic number of a neutral atom which has 51 neutrons and 40 electrons?
A. 40
B. 51
C. 91
D. 131
▶️Answer/Explanation
A
As atomic number of a neutral atom is equal to the number of electrons, its atomic number is 40.
Hence, correct answer is A.
Question
What is the relative atomic mass of an element with the following mass spectrum?
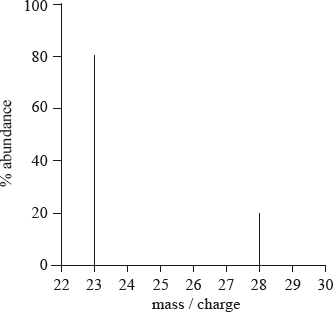
A. 24
B. 25
C. 26
D. 27
▶️Answer/Explanation
A
\(Relative Atomic mass = \frac{23\times 80 + 28\times 20}{100}\)
Relative Atomic mass = 24
Hence, correct answer is A.
Question
How many electrons does the ion \(_{{\text{15}}}^{{\text{31}}}{{\text{P}}^{3 – }}\) contain?
A. 12
B. 15
C. 16
D. 18
▶️Answer/Explanation
D
Here, 31 is the mass number, 15 in the atomic number and -3 is the charge on the atom.
Since atomic number is equal to the number of electrons on neutral atom, neutral P atom would have had 15 electrons.
But here we also have a negative charge of 3. So, total number of electrons = 15+3 = 18.
Hence, correct answer is D.
Question
Which statement about the species \(^{{\text{63}}}{\text{C}}{{\text{u}}^{2 + }}\) and \(^{{\text{65}}}{\text{C}}{{\text{u}}^ + }\) is correct?
A. Both species have the same number of protons.
B. Both species have the same number of electrons.
C. Both species have the same number of neutrons.
D. Both species have the same electron arrangement.
▶️Answer/Explanation
A
Cu has atomic number 29.
For \(^{{\text{63}}}{\text{C}}{{\text{u}}^{2 + }}\)
It has a positive charge of 2.
Number of electrons = Atomic number – Positive charge = 29-2 = 27.
Number of Protons = Atomic number = 29.
Atomic mass = Number of Protons + number of Neutrons
Number of Neutrons = 63 – 29 = 34.
Similarly, For \(^{{\text{65}}}{\text{C}}{{\text{u}}^ + }\)
It has a positive charge of 1.
Number of electrons = Atomic number – Positive charge = 29-1 = 28.
Number of Protons = Atomic number = 29.
Atomic mass = Number of Protons + number of Neutrons
Number of Neutrons = 65 – 29 = 36
Hence, both species have same number of Protons.
Hence, A is the correct answer.
Question
Which statement about the isotopes of nitrogen is correct?
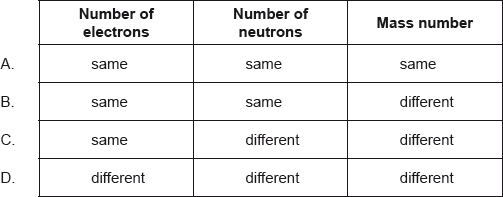
▶️Answer/Explanation
C
Isotopes have same atomic number but different mass number. Hence, they have equal number of protons and electrons and different mass number.
Nitrogen has two stable isotopes N-14 and N-15. Number of electrons and neutrons is same i.e. 7. Whereas, N-14 has 14 atomic mass and N-15 has 15 atomic mass.
