Question
a.Strontium exists as four naturally-occurring isotopes. Calculate the relative atomic mass of strontium to two decimal places from the following data.
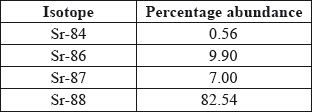 [2]
[2]
b.i.Define the term first ionization energy.[1]
b.ii.State what is meant by the term periodicity.[1]
b.iii.State the electron arrangement of argon. And iv. Explain why the noble gases, helium, neon and argon show the highest first ionization energies for their respective periods.[3]
b.v.A graph of atomic radius plotted against atomic number shows that the atomic radius decreases across a period. Explain why chlorine has a smaller atomic radius than sodium.[1]
b.vi.Explain why a sulfide ion, \({{\text{S}}^{2 – }}\), is larger than a chloride ion, \({\text{C}}{{\text{l}}^ – }\).[1]
b.vii.Explain why the melting points of the Group 1 metals \({\text{(Li}} \to {\text{Cs)}}\) decrease down the group whereas viii. The melting points of the Group 7 elements \({\text{(F}} \to {\text{I)}}\) increase down the group.[3]
▶️Answer/Explanation
Markscheme
a. \({A_{\text{r}}} = \frac{{[(0.56 \times 84) + (9.90 \times 86) + (7.00 \times 87) + (82.54 \times 88)]}}{{100}}\);
\( = 87.71\);
Award [1 max] if answer not given to two decimal places.
Award [2] for correct final answer.
Apply –1(U) if answer quoted in g or g\(\,\)mol–1.
first ionization energy: \({\text{M(g)}} \to {{\text{M}}^ + }{\text{(g)}} + {{\text{e}}^ – }{\text{/e}}\) / the (minimum) energy (\({\text{in kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}\)) to remove one electron from a gaseous atom / the energy required to remove one mole of electrons from one mole of gaseous atoms;
b.ii. periodicity: repeating pattern of (physical and chemical) properties;
2.8.8/sp version;
b.iv. Accept any two of the following:
the outer energy level/shell is full;
the increased charge on the nucleus;
great(est) attraction for electrons;
17 p in Cl nucleus attract the outer level more than 11 p in Na nucleus / greater nuclear charge attracts outer level more;
Allow converse for Na.
Do not accept larger nucleus.
\({{\text{S}}^{2 – }}\) has one proton less/ smaller nuclear charge so outer level held less strongly / OWTTE;
Allow converse for chloride.
Do not accept larger nucleus.
the radii of the metal atoms increase (from \({\text{Li}} \to {\text{Cs}}\)) (so the forces of attraction are less between them) / OWTTE;
b.viii.
the forces of attraction between halogen molecules are van der Waals;
forces increase with increasing mass/number of electrons;
Detailed Solution:
a. Relative atomic mass = Sum of products of isotope mass and fraction abundance
Relative atomic mass of Sr = 84*.0056+86*.0990+87*.07+88*.8254 = 87.71
b.
i.
The first ionization energy is defined as the energy required to remove the outermost (most loosely held) electron from a neutral, gaseous atom to form a positively charged ion (cation). It is a measure of the strength of attraction between the nucleus and the valence electron(s) of an atom.
Mathematically, the first ionization energy can be represented by the following equation:
X(g) → X^+(g) + e^-
ii.
Periodicity refers to the repeating pattern of properties and behaviors of elements in the periodic table. It is the fundamental concept that underlies the organization of the elements based on their atomic number and electron configuration.
The periodicity of elements arises from the periodic variation of their electron structure, which determines their chemical and physical properties. Elements within the same group (column) of the periodic table share similar characteristics due to having the same number of valence electrons, which determines their reactivity and chemical behavior.
iii.
In order to write the Argon electron configuration we first need to know the number of electrons for the Ar atom (there are 18 electrons). When we write the configuration we’ll put all 18 electrons in orbitals around the nucleus of the Argon atom.
The Argon electron configuration will be 1s22s22p63s23p6.
iv. The noble gases helium, neon, and argon have the highest first ionization energies in their respective periods due to their stable and fully filled electron configurations, making it energetically unfavorable to remove an electron from them.
v.
Chlorine (Cl) has a smaller atomic radius than sodium (Na) due to two main factors: effective nuclear charge and the number of electron shells.
Effective nuclear charge refers to the attractive force experienced by the outermost electrons of an atom from the nucleus. In chlorine, the effective nuclear charge is higher than in sodium. Although both elements have the same number of electron shells (3), the atomic number of chlorine (17) is higher than that of sodium (11), meaning it has more protons in the nucleus. The increased positive charge in the nucleus of chlorine attracts the electrons more strongly, resulting in a smaller atomic radius compared to sodium.
Additionally, the number of electron shells is the same for both chlorine and sodium, but chlorine has more electrons in its outermost shell. Chlorine has seven valence electrons in its 3rd energy level (3s^2 3p^5), while sodium has only one valence electron in its 3rd energy level (3s^1). The increased electron-electron repulsion in the outermost shell of chlorine causes the electrons to be slightly pushed away from each other, leading to a smaller atomic radius compared to sodium.
vi.
The sulfide ion (S2-) is larger than the chloride ion (Cl-) due to the difference in the effective nuclear charge.
The effective nuclear charge is the net positive charge experienced by the outermost electrons of an ion. In the case of the sulfide ion, it has 16 protons in its nucleus, while the chloride ion has 17 protons. Both ions have gained two electrons to achieve a stable electron configuration. However, the sulfide ion has one less proton compared to the chloride ion, resulting in a lower effective nuclear charge. This lower effective nuclear charge leads to weaker attraction between the nucleus and the outermost electrons in the sulfide ion, causing it to have a larger atomic radius compared to the chloride ion.
vii.
The melting points of Group 1 metals decrease down the group due to atomic Size. As you move down Group 1, the atomic size or radius of the elements increases. This is because each successive element has an additional energy level of electrons. The increase in atomic size leads to weaker metallic bonding between the atoms, making it easier to overcome the attractive forces and melt the metal.
viii. The increasing melting points of Group 7 elements (halogens) down the group are primarily due to the increasing strength of van der Waals forces, specifically London dispersion forces, which are a type of intermolecular force. With larger atoms and more electrons, there is an increase in the electron cloud’s size and polarizability. The larger and more polarizable electron cloud leads to stronger London dispersion forces between the atoms or molecules. These stronger intermolecular forces require more energy to overcome, resulting in higher melting points down the group.
Question
Explain why:
a.i.Define the term first ionization energy.[2]
a.ii.Explain why the first ionization energy of magnesium is higher than that of sodium.[2]
b.i.calcium has a higher melting point than potassium.[2]
b.ii.sodium oxide has a higher melting point than sulfur trioxide.[3]
c.i.Define the terms acid and base according to the Brønsted-Lowry theory and state one example of a weak acid and one example of a strong base.[2]
c.ii.Describe two different methods, one chemical and one physical, other than measuring the pH, that could be used to distinguish between ethanoic acid and hydrochloric acid solutions of the same concentration.[4]
c.iii.Black coffee has a pH of 5 and toothpaste has a pH of 8. Identify which is more acidic and deduce how many times the \({\text{[}}{{\text{H}}^ + }{\text{]}}\) is greater in the more acidic product.[2]
d.Samples of i. sodium oxide and ii. sulfur trioxide are added to separate beakers of water. Deduce the equation for each reaction and identify each oxide as acidic, basic or neutral.[3]
▶️Answer/Explanation
Markscheme
a.i.
the amount of energy required to remove one (mole of) electron(s);
from (one mole of) an atom(s) in the gaseous state;
greater positive charge on nucleus / greater number of protons / greater core charge;
greater attraction by Mg nucleus for electrons (in the same shell) / smaller atomic radius;
calcium ionic charge is twice/greater than the potassium ionic charge / calcium has more delocalized electrons than potassium;
greater attraction of delocalized electrons and \({\text{C}}{{\text{a}}^{2 + }}\) / less attraction between the delocalized electrons and \({{\text{K}}^ + }\);
Do not accept calcium ion has a 2+ without comparison to \({{\text{K}}^ + }\).
Na2O ionic/(stronger electrostatic) attractions between \({\text{N}}{{\text{a}}^ + }\) and \({{\text{O}}^{2 – }}\);
\({\text{S}}{{\text{O}}_{\text{3}}}\) has (weak) intermolecular/van der Waals’/London/dispersion/dipoledipole attractions;
intermolecular/van der Waals’/London/dispersion/dipole-dipole forces are weaker/more easily broken than (strong) ionic bonds / ionic bonds are stronger/harder to break than intermolecular bond/van der Waals’/London/dispersion/dipole-dipole forces;
acid is a proton/\({{\text{H}}^ + }\) donor and base is a proton/\({{\text{H}}^ + }\) acceptor;
\({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\)/\({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) and NaOH/KOH/\({\text{Ba(OH}}{{\text{)}}_{\text{2}}}\);
Accept any suitable examples.
Chemical
reaction with reactive metal/Mg/Zn/carbonate/hydrogen carbonate;
hydrochloric acid would react faster/more vigorously / ethanoic acid would react slower/less vigorously;
OR
react with alkali;
temperature change will be more for hydrochloric acid / temperature change will be less for ethanoic acid;
Physical
conductivity;
hydrochloric acid will conduct more/higher / ethanoic acid will conduct less/lower;
Accept other suitable examples.
black coffee;
\({\text{1}}{{\text{0}}^{\text{3}}}\)/1000 times;
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O(s)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{2NaOH(aq)}}\);
\({\text{S}}{{\text{O}}_3}{\text{(l)}} + {{\text{H}}_2}{\text{O(l)}} \to {{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}}\);
Ignore state symbols.
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\): basic and \({\text{S}}{{\text{O}}_{\text{3}}}\): acidic;
Detailed Explanation:
a.
i.
The first ionization energy is defined as the energy required to remove the outermost (most loosely held) electron from a neutral, gaseous atom to form a positively charged ion (cation). It is a measure of the strength of attraction between the nucleus and the valence electron(s) of an atom.
Mathematically, the first ionization energy can be represented by the following equation:
X(g) → X^+(g) + e^-
ii. Magnesium has a higher effective nuclear charge than sodium. Both elements have the same number of energy levels (valence shell), but magnesium has one more proton in its nucleus compared to sodium. The higher positive charge in the nucleus of magnesium attracts its electrons more strongly, making it more difficult to remove an electron and resulting in a higher first ionization energy.
b.
i. Calcium has a higher atomic number and more protons in its nucleus compared to potassium. The higher effective nuclear charge in calcium results in stronger attraction between the nucleus and the electrons, leading to stronger metallic bonding. This stronger metallic bonding requires more energy to break, resulting in a higher melting point.
Calcium has an electron configuration of [Ar] 4s², while potassium has an electron configuration of [Ar] 4s¹. Both elements have their outermost electrons in the 4s orbital. The presence of two electrons in the outermost shell of calcium provides a more stable electron configuration compared to potassium, which has only one electron. The increased stability in calcium contributes to stronger metallic bonding and a higher melting point.
ii. Sodium oxide is an ionic compound formed by the combination of sodium cations (Na+) and oxide anions (O2-). Ionic compounds generally have high melting points due to the strong electrostatic attractions between the oppositely charged ions.
On the other hand, sulfur trioxide is a covalent compound where sulfur and oxygen atoms share electrons to form covalent bonds. Covalent compounds tend to have lower melting points compared to ionic compounds because the intermolecular forces between molecules (in this case, van der Waals forces) are generally weaker than the electrostatic attractions in ionic compounds.
c.
i. As per the Brønsted-Lowry theory, Acid is a proton/\({{\text{H}}^ + }\) donor and Base is a proton/\({{\text{H}}^ + }\) acceptor.
Examples of acids : \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\),\({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) etc.
Examples of bases : NaOH,KOH,\({\text{Ba(OH}}{{\text{)}}_{\text{2}}}\) etc.
ii. Chemical method: Reaction with Sodium Carbonate.
Ethanoic acid (acetic acid) reacts with sodium carbonate to produce carbon dioxide gas. This reaction can be used to differentiate between ethanoic acid and hydrochloric acid solutions. If effervescence (bubbling) occurs in the ethanoic acid solution but not in the hydrochloric acid solution, it indicates the presence of ethanoic acid. Carbon dioxide gas is produced when ethanoic acid reacts with sodium carbonate.
Physical method: The hydrochloric acid solution is expected to have a higher conductivity compared to the ethanoic acid solution. This is because hydrochloric acid is a strong acid that ionizes almost completely in water, producing a high concentration of ions (H+ and Cl-) that contribute to the electrical conductivity. Ethanoic acid, being a weak acid, ionizes only partially, resulting in a lower concentration of ions and lower conductivity.
iii. Black coffee pH =5, \({\text{[}}{{\text{H}}^ + }{\text{]}}\) = 10-5
Toothpaste pH=8, \({\text{[}}{{\text{H}}^ + }{\text{]}}\) = 10-8
