Question
There has been significant growth in the use of carbon nanotubes, CNT.
a. Explain these properties of carbon nanotubes.
b(i)Alloying metals changes their properties. Suggest one property of magnesium that could be improved by making a magnesium-CNT alloy.
b(iipure magnesium needed for making alloys can be obtained by electrolysis of molten magnesium chloride.
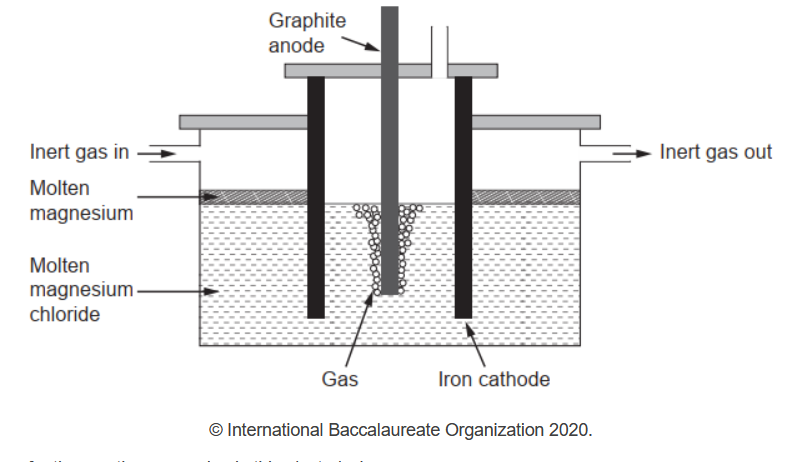
Write the half-equations for the reactions occurring in this electrolysis.
$\mathrm{b}$ (iiifalculate the theoretical mass of magnesium obtained if a current of $3.00 \mathrm{~A}$ is used for 10.0 hours. Use charge $(Q)=\operatorname{current}(I) \times \operatorname{time}(t)$ and section 2 of the data booklet
b(iks.uggest a gas which should be continuously passed over the molten magnesium in the electrolytic cell.
c. Zeolites can be used as catalysts in the manufacture of CNT. Explain, with reference to their structure, the high selectivity of zeolites.
d. Experiments have been done to explore the nematic liquid crystal behaviour of CNT. Justify how CNT molecules could be classified as nematic.
▶️Answer/Explanation
Markscheme
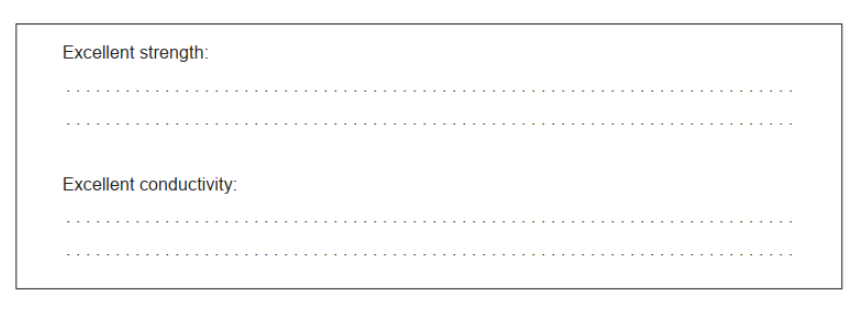
a. Excellent strength: defect-free $A N D$ rigid/regular 2D/3D
Excellent conductivity: delocalized electrons
Accept “carbons/atoms are all covalently bonded to each other” for M1.
b(i)Any of:
ductility
strength/resistance to deformation
malleability
hardness
resistance to corrosion/chemical resistance
range of working temperatures
density
Do not accept “conductivity”.
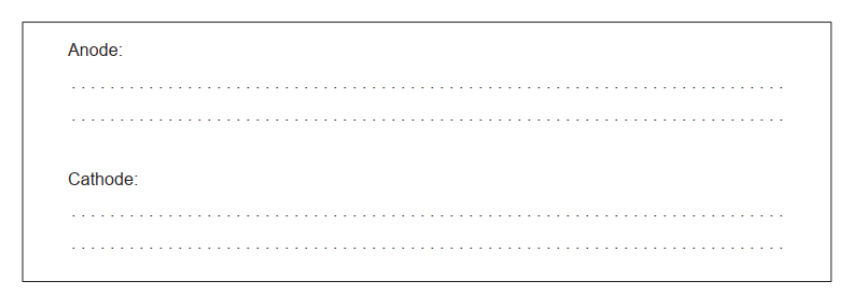
b(ii)Anode: $2 \mathrm{Cl}^{-} \rightarrow \mathrm{Cl}_2(\mathrm{~g})+2 \mathrm{e}^{-}$
Cathode: $\mathrm{Mg}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Mg}(1)$
Accept $\mathrm{Cl}^{-} \rightarrow \mathrm{Cl}_2(\mathrm{~g})+e^{-}$.
Award [1 max] for correct equations at incorrect electrodes.
$$
\mathrm{b}(\mathrm{iii}) Q=I \times t=3.00 \times 10.0 \times 3600=» 108000 \mathrm{C} \boldsymbol{V}
$$
$$
\begin{aligned}
& « \frac{Q}{F}=\frac{108000 \mathrm{C}}{96500 \mathrm{C} \mathrm{mol}^{-1}}=» 1.12 \ll \mathrm{mol} \mathrm{e}^{-} » \\
& « \frac{1.12 \mathrm{~mol}}{2}=0.560 \mathrm{~mol} \mathrm{Mg} » \\
& « m=0.560 \mathrm{~mol} \times 24.31 \mathrm{~g} \mathrm{~mol}{ }^{-1}=» 13.6 \ll \mathrm{g} »
\end{aligned}
$$
Award [3] for correct final answer.
b(ivergon/Ar/helium/He
Accept any identified noble/inert gas.
Accept name OR formula.
Do not accept “nitrogen $/ \mathrm{N}_2$ “.
c. pores/cavities/channels/holes/cage-like structures
«only» reactants with appropriate/specific size/geometry/structure fit inside/go through/are activated/can react
d. rod-shaped molecules
OR
«randomly distributed but» generally align
OR
no positional order $\boldsymbol{A N D}$ have «some» directional order/pattern
Accept “linear” for “rod-shaped”.
