Question
What are the correct formulas of the following ions?
Sulphide, Bromide and Nitride.
A. S–, Br-2 and N-3
B. S-2, Br-2 and N-3
C. S–, Br– and N-3
D. S-2, Br– and N-3
▶️Answer/Explanation
D
Sulphide is S-2
Bromide is Br–
and Nitride is N-3 .
Question
Which is the best description of ionic bonding?
A. The electrostatic attraction between positively charged nuclei and an electron pair
B. The electrostatic attraction between positive ions and delocalized negative ions
C. The electrostatic attraction between positive ions and delocalized electrons
D. The electrostatic attraction between oppositely charged ions
▶️Answer/Explanation
D
Ionic bonding occurs due to the electrostatic attraction between oppositely charged ions i.e. positive and negative ions.
Question
Which statement best describes the intramolecular bonding in HCN(l)?
A. Electrostatic attractions between \({{\text{H}}^ + }\) and \({\text{C}}{{\text{N}}^ – }\) ions
B. Only van der Waals’ forces
C. Van der Waals’ forces and hydrogen bonding
D. Electrostatic attractions between pairs of electrons and positively charged nuclei
▶️Answer/Explanation
D
HCN has Electrostatic attractions between pairs of electrons of \({\text{C}}{{\text{N}}^ – }\) ion and positively charged nuclei of \({{\text{H}}^ + }\) .
Question
What are the correct formulas of the following ions?
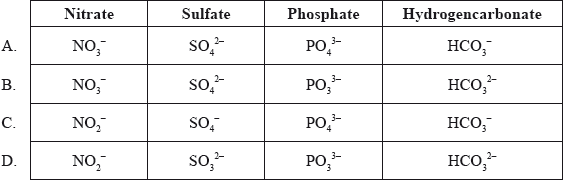
▶️Answer/Explanation
A
SO32- is sulfite ion. NO2– is nitrite ion.
Correct answer is A.
Question
Which combination best describes the type of bonding present and the melting point of silicon dioxide respectively?
A. Ionic, High
B. Ionic, Low
C. Covalent, High
D. Covalent, Low
▶️Answer/Explanation
C
In silicon dioxide, each silicon atom forms four covalent bonds with four oxygen atoms. In silica, each silicon atom shares electrons with four oxygen atoms. This means that each silicon atom forms a single covalent bond with four oxygen atoms.
Silicon Dioxide has a high melting and boiling point. The many covalent bonds in silica are very strong, therefore a large amount of energy is needed to break them therefore a high temperature is required.
Question
The formula of gallium phosphate is \({\text{GaP}}{{\text{O}}_{\text{4}}}\). What is the correct formula of gallium sulfate?
A. \({\text{GaS}}{{\text{O}}_{\text{4}}}\)
B. GaS
C. \({\text{G}}{{\text{a}}_{\text{2}}}{{\text{(S}}{{\text{O}}_{\text{4}}}{\text{)}}_{\text{3}}}\)
D. \({\text{G}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{3}}}\)
▶️Answer/Explanation
C
We know that Phosphate is PO4-3. Hence, GaPO4 has Ga+3.
Sulphate is SO4-2. Therefore to form Gallium Sulphate, we require, say x SO4-2 ions and y Ga+3 ions.
Now, for charge balancing, x(-2)+y(+3) = 0
2x=3y
Take x=3, y=2.
Formula for Gallium Sulphate would become \({\text{G}}{{\text{a}}_{\text{2}}}{{\text{(S}}{{\text{O}}_{\text{4}}}{\text{)}}_{\text{3}}}\) .
