Question
a. Draw the Lewis structures for (i) carbon monoxide, CO, (ii) carbon dioxide, \({\text{C}}{{\text{O}}_{\text{2}}}\) and (iii) methanol, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\).
List, with an explanation, the three compounds in order of increasing carbon to oxygen bond length (shortest first).
\({\text{CO}}_3^{2 – }\)
\({\text{BF}}_4^ – \)
Define a Brønsted-Lowry acid.
Deduce the two acids and their conjugate bases in the following reaction:
\[{{\text{H}}_2}{\text{O(l)}} + {\text{N}}{{\text{H}}_3}{\text{(aq)}} \rightleftharpoons {\text{O}}{{\text{H}}^ – }{\text{(aq)}} + {\text{NH}}_4^ + {\text{(aq)}}\]
Define the term weak acid and state the equation for the reaction of ethanoic acid with water.
Vinegar, which contains ethanoic acid, can be used to clean deposits of calcium carbonate from the elements of electric kettles. State the equation for the reaction of ethanoic acid with calcium carbonate.
▶️Answer/Explanation
Markscheme
a. i, ii and iii.
All outer electron pairs must be shown for mark in each case.
Accept electrons shown as all rather than \( \bullet \) and x.
\({\text{CO}} < {\text{C}}{{\text{O}}_{\text{2}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\);
triple bonds are shorter than double bonds which are shorter than single bonds / the more pairs of electrons that are shared the stronger the attracting so the shorter the bond / OWTTE;
The order must be correct to gain the second marking point unless ECF from (a).
\({\text{(C}}{{\text{O}}_{\text{2}}}{\text{)}}\)linear;
180°;
\({\text{(CO}}_3^{2 – }{\text{)}}\) trigonal planar/triangular planar;
120°;
\({\text{(BF}}_4^ – {\text{)}}\) tetrahedral;
109.5° / 109° / 109° \(28’\);
donates a proton / \({{\text{H}}^ + }\) ion;
\[\begin{array}{*{20}{c}} {{\text{(acid)}}}&{{\text{(conjugate base)}}} \\ {{{\text{H}}_{\text{2}}}{\text{O}}}&{{\text{O}}{{\text{H}}^ – }{\text{;}}} \\ {{\text{NH}}_4^ + }&{{\text{N}}{{\text{H}}_{\text{3}}}{\text{;}}} \end{array}\]
[1 max] if all four acids and bases given but not clearly paired.
partially dissociated or ionized;
\({\text{C}}{{\text{H}}_3}{\text{COOH}} + {{\text{H}}_2}{\text{O}} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ – } + {{\text{H}}_3}{{\text{O}}^ + }/{\text{C}}{{\text{H}}_3}{\text{COOH}} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ – } + {{\text{H}}^ + }\);
\( \rightleftharpoons \) required for mark.
\({\text{2C}}{{\text{H}}_3}{\text{COOH}} + {\text{CaC}}{{\text{O}}_3} \to {\text{Ca(C}}{{\text{H}}_3}{\text{COO}}{{\text{)}}_2} + {\text{C}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}}\)
Award [1] for correct reactants and products and [1] for balancing.
Detailed Solution:
(i) 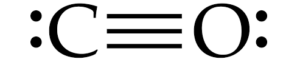
(ii) 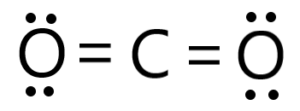
(iii) 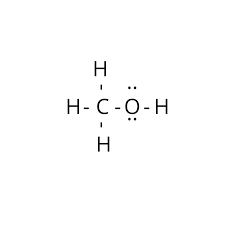
(iv) In carbon monoxide, the carbon atom is triple-bonded to the oxygen atom. The carbon-to-oxygen bond in CO is a triple bond, consisting of one sigma bond and two pi bonds. The triple bond in CO is the shortest and strongest carbon-to-oxygen bond among the three compounds.
In carbon dioxide, the central carbon atom is double-bonded to two oxygen atoms. The carbon-to-oxygen bond in CO2 is a double bond, consisting of one sigma bond and one pi bond. The double bond in CO2 is longer than a triple bond but shorter than a single bond.
In methanol, the carbon atom is single-bonded to one oxygen atom, forming a carbon-to-oxygen single bond. Single bonds are longer than double or triple bonds. Therefore, the carbon-to-oxygen bond in CH3OH is the longest among the three compounds.
Order of increasing carbon to oxygen bond length : CO<CO2<CH3OH
b.
(i) 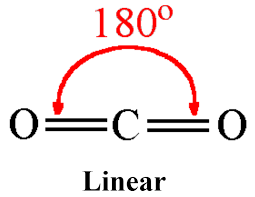
(ii) 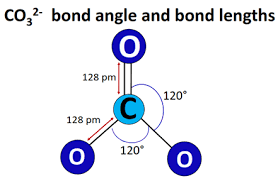 It is trigonal planar in shape.
It is trigonal planar in shape.
(iii) 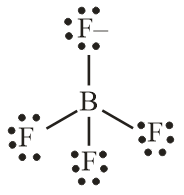 It is tetrahedral in shape with 109.5 degree bond angles.
It is tetrahedral in shape with 109.5 degree bond angles.
(iv) A Brønsted-Lowry acid is a substance that donates a proton (H+) to another substance in a chemical reaction.
c.
(i) \[{{\text{H}}_2}{\text{O(l)}} + {\text{N}}{{\text{H}}_3}{\text{(aq)}} \rightleftharpoons {\text{O}}{{\text{H}}^ – }{\text{(aq)}} + {\text{NH}}_4^ + {\text{(aq)}}\]
Here, Acid is H2O as it donates a proton (H+) . Its conjugate base is (OH-) ion.
Also, NH4+ is an acid and its conjugate base is NH3.
(ii) A weak acid is an acid that only partially dissociates in water, resulting in a relatively low concentration of hydrogen ions (H+). Unlike strong acids, which completely dissociate in water, weak acids establish an equilibrium between the undissociated acid molecules and their dissociated ions.
The equation for the reaction of ethanoic acid (also known as acetic acid) with water can be represented as follows:
CH3COOH + H2O ⇌ CH3COO- + H3O+
d.
(i)
The equation for the reaction of ethanoic acid (acetic acid) with calcium carbonate can be represented as follows:
2CH3COOH + CaCO3 -> Ca(CH3COO)2 + H2O + CO2
In this reaction, two molecules of ethanoic acid (CH3COOH) react with one molecule of calcium carbonate (CaCO3) to produce one molecule of calcium acetate (Ca(CH3COO)2), water (H2O), and carbon dioxide (CO2). The reaction involves the displacement of carbonate ions (CO3^2-) in calcium carbonate by the acetate ions (CH3COO-) from ethanoic acid.
Question
a.i. Ionic bonding and covalent bonding are two types of bonding. Ionic bonding occurs in sodium chloride. Describe what is meant by the term ionic bonding.
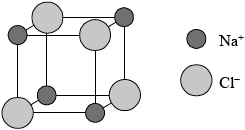
Sodium chloride has a lattice structure. Describe the lattice structure of sodium chloride including a suitable representative three-dimensional diagram. On the diagram, label each ion and distinguish between the different types of ions present using different sized spheres.
Ammonium phosphate is also an ionic compound, used in the manufacture of fertilizers. State the chemical formula of ammonium phosphate.
Deduce the Lewis (electron dot) structure and predict the shape of each molecule, using the valence shell electron pair repulsion theory (VSEPR).
State and explain the F–S–F bond angle in SF2.
Deduce whether each of the three molecules is polar or non-polar, giving your reason in each case.
b.ii. SF2:
b.iii. BF3:
b.iv. PCl3:
Using electronegativity values from Table 7 of the Data Booklet, state and explain which of the following compounds, IBr, BaCl2, CsI and HBr are ionic and which compounds are covalent.
b.v. IBr:
b.vi. BaCl2:
b.vii. CsI:
b.viii. HBr:
▶️Answer/Explanation
Markscheme
a.i.(electrostatic) attraction between oppositely charged ions/cation and anion/positive and negative ions;
Do not allow electrostatic attraction between metals and non-metals.
Description:
a lattice is a giant, regular/repeating arrangement/array;
of (chloride) anions/negative ions/\({\text{C}}{{\text{l}}^ – }\) and (sodium) cations/positive ions/\({\text{N}}{{\text{a}}^ + }\);
each sodium ion surrounded by six chloride ions / each chloride ion surrounded by six sodium ions;
M2 may also be scored from a diagrammatical key or labels on each ion.
M3 may also be scored by a correctly represented cubic representation showing the six-coordination around either the sodium ion or each chloride ion.
Diagram:
cubic lattice type representation (showing a minimum of one sub-cube and alternating \({\text{N}}{{\text{a}}^ + }\) and \({\text{C}}{{\text{l}}^ – }\) ions);
\({\text{C}}{{\text{l}}^ – }\) shown represented bigger than \({\text{N}}{{\text{a}}^ + }\) on diagram;
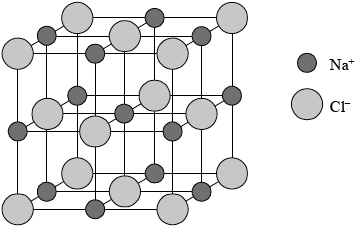
Award [4] for correctly drawn diagram (like above) with ions clearly identified.
Award [3 max] for the following diagram below if no explanation in words is given.
(NH4)3PO4;
Allow use of square brackets.
allow any bond angle in the range 97° to less than 109.5° (experimental value is 98°);
due to four negative charge centres/four electron pairs/four electron domains (two of which are lone pairs)/tetrahedral arrangement of electron pairs;
extra repulsion due to two lone pairs of electrons repelling each other / lone pairs occupy more space (than bonding pairs) so F–S–F bond angle decreases from 109.5° / OWTTE;
Answers which refer to electronegativity consideration of F’s also are correct, as long as LP/LP repulsion is also mentioned to score M3.
SF2:
polar because net dipole moment present in molecule / SF bond polarities do not cancel each other out / unsymmetrical distribution of charge / OWTTE;
b.iii.
BF3:
non-polar because no net dipole moment present in molecule / BF bond polarities do cancel each other out / symmetrical distribution of charge / OWTTE;
b.iv.
PCl3:
polar because net dipole moment present in molecule / PCl bond polarities do not cancel each other out / unsymmetrical distribution of charge / OWTTE;
Award [1 max] for SF2 polar, BF3 non-polar, PCl3 polar even if explanations are incorrect or are not given.
Polarity may also be explained using diagrams showing net dipole moments.
IBr:
\(\Delta \chi = (3.0 – 2.7) = 0.3\), covalent
b.vi.
BaCl2:
\(\Delta \chi = (3.2 – 0.9) = 2.3\), ionic
b.vii.
CsI:
\(\Delta \chi = (2.7 – 0.8) = 1.9\), ionic
b.viii.
HBr:
\(\Delta \chi = (3.0 – 2.2) = 0.8\), covalent
Award [2] for all four correct, [1] for two or three correct.
Award [1 max] for stating IBr, HBr covalent and BaCl2, CsI ionic.
Allow polar covalent instead of covalent.
Allow large electronegativity difference for ionic and small electronegativity difference for covalent.
Detailed Solution:
a.
(i) Ionic bonding is a type of chemical bonding that occurs between atoms with significantly different electronegativities. It involves the transfer of electrons from one atom to another, resulting in the formation of ions. The atoms involved in ionic bonding are typically a metal and a nonmetal.
In an ionic bond, the atom with a lower electronegativity (often a metal) loses one or more electrons to achieve a stable electron configuration and forms a positively charged ion called a cation. The atom with a higher electronegativity (often a nonmetal) gains these electrons to achieve a stable electron configuration and forms a negatively charged ion called an anion. The resulting electrostatic attraction between the oppositely charged ions holds them together and forms an ionic compound. The transfer of electrons leads to the formation of full outer electron shells for both ions, resulting in increased stability.
(ii) NaCl has a cubic unit cell. It is best thought of as a face-centered cubic array of anions with an interpenetrating fcc cation lattice (or vice-versa). The cell looks the same whether you start with anions or cations on the corners.
Lattice structure of NaCl is depicted as :

Its unit cell is : 
(iii) The chemical formula of ammonium phosphate is (NH4)3PO4
(iv) (v) (vi)

b.
(i) 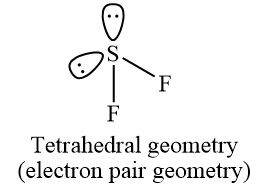
Since the central atom in SF2 has four regions of electron density(2 bond pairs + 2 lone pairs), its molecular geometry will be bent and its electron geometry will be tetrahedral.
The bond angle of SF2 is around 98º as due to the lone pair on the sulfur central atom, both fluorine atom pushes down in the downward direction that contract the angle between them, and gives the approx F-S-F 98º angle.
(ii) SF2 is polar because:
In SF2, the sulfur atom is bonded to two fluorine atoms. Fluorine is more electronegative than sulfur, resulting in a polarity difference between the two atoms. As a result, the sulfur-fluorine bonds in SF2 are polar covalent bonds, with fluorine being slightly more negative (δ-) and sulfur being slightly more positive (δ+).
To determine the overall polarity of SF2, we need to consider its molecular geometry. SF2 has a bent or V-shaped molecular geometry due to the presence of two lone pairs of electrons on the sulfur atom. The fluorine atoms are arranged in a bent shape around the central sulfur atom, causing an uneven distribution of electron density.
The combination of polar bonds and the bent molecular geometry results in an overall dipole moment in SF2. The dipole moments of the polar bonds do not cancel each other out due to the asymmetric shape of the molecule. As a result, SF2 has a net molecular dipole, making it a polar molecule.
(iii) BF3 has a trigonal planar molecular geometry, where the three fluorine atoms are symmetrically arranged around the central boron atom. The angles between the boron-fluorine bonds are 120 degrees.
In a trigonal planar arrangement, the bond polarities can cancel each other out, resulting in a nonpolar molecule. In the case of BF3, the bond polarities of the three boron-fluorine bonds are pointing in the same direction but are balanced symmetrically around the central boron atom.
As a result, the dipole moments of the individual polar bonds cancel each other out, resulting in a net dipole moment of zero. This makes BF3 a nonpolar molecule.
(iv) PCl3 has a trigonal pyramidal molecular geometry, with the three chlorine atoms positioned around the central phosphorus atom and one lone pair of electrons on phosphorus. The bond angles between the phosphorus-chlorine bonds are approximately 107 degrees.
In a trigonal pyramidal arrangement, the lone pair of electrons and the polar bond dipole moments do not cancel each other out due to the asymmetric shape of the molecule. The lone pair of electrons creates an uneven distribution of electron density, causing the molecule to have a net molecular dipole moment.
Therefore, even though the individual phosphorus-chlorine bonds are polar, the molecular geometry of PCl3 results in an overall molecular dipole, making it a polar molecule.
(v) IBr is covalent because \(\Delta \chi = (3.0 – 2.7) = 0.3\),
When the electronegativity difference between two atoms is relatively small (less than 1.7), a covalent bond is generally formed. In a covalent bond, the atoms share electrons to achieve a stable electron configuration. This occurs when two nonmetals or a nonmetal and a metalloid bond together.
(vi) BaCl2 is ionic because \(\Delta \chi = (3.2 – 0.9) = 2.3\),
when the electronegativity difference between two atoms is relatively large (greater than 1.7), an ionic bond is typically formed. In an ionic bond, there is a complete transfer of electrons from one atom to another, resulting in the formation of positively charged ions (cations) and negatively charged ions (anions). This type of bond generally occurs between a metal and a nonmetal.
(vii) CsI is ionic because \(\Delta \chi = (2.7 – 0.8) = 1.9\),
when the electronegativity difference between two atoms is relatively large (greater than 1.7), an ionic bond is typically formed. In an ionic bond, there is a complete transfer of electrons from one atom to another, resulting in the formation of positively charged ions (cations) and negatively charged ions (anions). This type of bond generally occurs between a metal and a nonmetal.
(viii) HBr is covalent because \(\Delta \chi = (3.0 – 2.2) = 0.8\),
When the electronegativity difference between two atoms is relatively small (less than 1.7), a covalent bond is generally formed. In a covalent bond, the atoms share electrons to achieve a stable electron configuration. This occurs when two nonmetals or a nonmetal and a metalloid bond together.
Question
a.i.Define the term isotopes.[1]
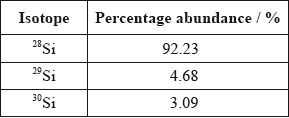
Calculate the relative atomic mass of silicon using this data.[2]
▶️Answer/Explanation
Markscheme
a.i. atoms of the same element with the same atomic number/Z/same number of protons, but different mass numbers/A/different number of neutrons;
\((0.9223 \times 28) + (0.0468 \times 29) + (0.0309 \times 30)\);
28.1/28.11;
Working must be shown to get [2], do not accept 28.09 on its own (given in the data booklet).
Silicon dioxide
single covalent (bonds);
network/giant covalent/ macromolecular / repeating tetrahedral units;
a.iv.
Carbon dioxide
double covalent (bonds);
(simple / discrete) molecular;
Marks may be obtained from suitable structural representations of SiO2 and CO2.
 ;
;
Allow crosses or dots for lone-pair.
trigonal/triangular pyramidal;
b.ii (\( \sim \))107° / less than 109.5°;
Do not allow ECF.
LP-BP repulsion \( > \) BP-BP repulsion / one lone pair and three bond pairs / lone pairs/non-bonding pairs repel more than bonding-pairs;
Do not accept repulsion between atoms.
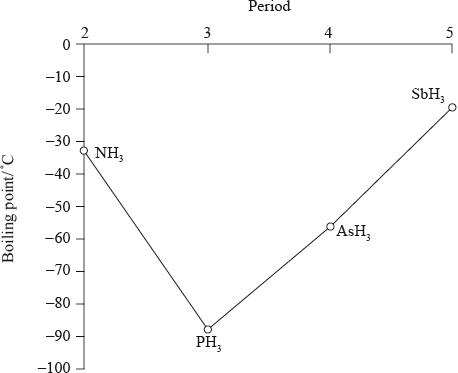
boiling points increase going down the group (from \({\text{P}}{{\text{H}}_{\text{3}}}\) to \({\text{As}}{{\text{H}}_{\text{3}}}\) to \({\text{Sb}}{{\text{H}}_{\text{3}}}\));
\({M_{\text{r}}}\)/number of electrons/molecular size increases down the group;
Accept electron cloud increases down the group for the second marking point.
greater dispersion/London/van der Waal’s forces;
\({\text{N}}{{\text{H}}_{\text{3}}}\)/ammonia has a higher boiling point than expected due to the hydrogen bonding between the molecules;
Do not accept hydrogen bonding alone.
CO:
![]()
Award [1] for showing the net dipole moment, or explaining it in words (unsymmetrical distribution of charge).
c.ii. \(N{O_2}\):
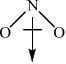
Award [1] for correct representation of the bent shape and [1] for showing the net dipole moment, or explaining it in words (unsymmetrical distribution of charge).
c.iii. \(C{O_2}\):
![]()
Award [1] for correct representation of the linear shape and [1] for showing the two equal but opposite dipoles or explaining it in words (symmetrical distribution of charge).
For all three molecules, allow either arrow or arrow with bar for representation of dipole moment.
Allow correct partial charges instead of the representation of the vector dipole moment.
Ignore incorrect bonds.
Lone pairs not needed.
Detailed Solution:
a.
i. Isotopes are variants of an element that have the same number of protons in the nucleus but differ in the number of neutrons. In other words, isotopes are different forms of an element that have the same atomic number (number of protons) but different atomic masses (sum of protons and neutrons).
ii. Relative atomic mass = (Sum of the product of isotope mass and fraction abundance)
Relative atomic mass = 28*0.0223+29*0.0468+30*0.0309 = 28.1.
iii.
Silicon dioxide (SiO2) is a chemical compound commonly known as silica or quartz. It is a covalent compound that consists of one silicon atom bonded to two oxygen atoms. The structure of silicon dioxide can be described as a three-dimensional network of interconnected silicon and oxygen atoms.
In the crystal lattice of silicon dioxide, each silicon atom is surrounded by four oxygen atoms in a tetrahedral arrangement. Each oxygen atom is shared between two silicon atoms, forming strong covalent bonds. This results in a highly stable and rigid structure.
![]()
iv.
The structure of carbon dioxide (CO2) can be described as a linear molecule with a carbon atom in the center bonded to two oxygen atoms. Each oxygen atom is connected to the carbon atom by a double bond.
In the Lewis structure representation, the carbon atom is in the middle and has two electron pairs and two oxygen atoms around it. Each oxygen atom shares two electrons with the carbon atom through a double bond, resulting in a total of four shared electrons. The remaining two electron pairs on each oxygen atom are unshared (also known as lone pairs).
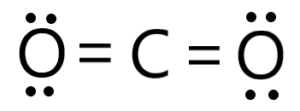
b.
i.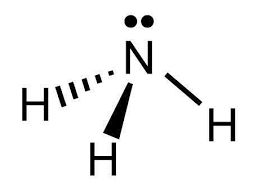
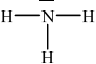
The shape of ammonia (NH3) is trigonal pyramidal.
In ammonia, the central nitrogen atom is bonded to three hydrogen atoms. The nitrogen atom has one lone pair of electrons, in addition to the three bonding pairs of electrons. The three hydrogen atoms are positioned around the nitrogen atom, forming a triangular base.
The lone pair of electrons on nitrogen affects the molecular geometry, pushing the bonding electron pairs slightly closer to each other. This results in a distorted tetrahedral arrangement, where the three hydrogen atoms and the lone pair of electrons are not symmetrically arranged around the nitrogen atom.
ii. The bond angles between the hydrogen-nitrogen-hydrogen atoms are approximately 107 degrees, slightly less than the ideal tetrahedral angle of 109.5 degrees, due to the repulsion between the lone pair and bonding pairs of electrons.
iii.
PH3 < NH3
The lower boiling point of ammonia compared to phosphine is primarily due to the differences in molecular polarity and hydrogen bonding.
Ammonia (NH3) has a higher boiling point than expected based on its molecular weight alone because it can form hydrogen bonds. In ammonia, the electronegative nitrogen atom can attract the hydrogen atoms of neighboring molecules, resulting in intermolecular hydrogen bonding. These hydrogen bonds are relatively strong and require more energy to break, resulting in a higher boiling point for ammonia.
Phosphine (PH3), on the other hand, is less polar and exhibits weaker intermolecular forces compared to ammonia. While phosphine molecules can form hydrogen bonds, the electronegativity difference between phosphorus and hydrogen is smaller than that between nitrogen and hydrogen. As a result, the strength of hydrogen bonding in phosphine is weaker than in ammonia, leading to a lower boiling point for phosphine.
PH3 < AsH3 < SbH3
PH3 (Phosphine): Phosphine molecules have a dipole moment due to the electronegativity difference between phosphorus and hydrogen. However, the dipole moment in phosphine is relatively weak. Additionally, phosphine experiences relatively weak London dispersion forces between molecules. As a result, the intermolecular forces in phosphine are weaker overall, leading to a lower boiling point compared to the other compounds.
AsH3 (Arsine): Arsine molecules have a larger dipole moment compared to phosphine due to the electronegativity difference between arsenic and hydrogen. This increased dipole moment enhances the strength of dipole-dipole interactions between arsine molecules. As a result, arsine has stronger intermolecular forces compared to phosphine, leading to a higher boiling point.
SbH3 (Stibine): Stibine molecules have an even larger dipole moment than arsine due to the electronegativity difference between antimony and hydrogen. The increased dipole moment in stibine further enhances the strength of dipole-dipole interactions compared to arsine. Additionally, stibine experiences stronger London dispersion forces due to the larger, more polarizable antimony atom. These stronger intermolecular forces result in a higher boiling point for stibine compared to both arsine and phosphine.
c.
i. ![]()
CO (carbon monoxide) is a polar molecule due to the difference in electronegativity between carbon and oxygen atoms and the molecular geometry.
In a CO molecule, oxygen is more electronegative than carbon, meaning it has a higher affinity for electrons. As a result, the shared electrons in the carbon-oxygen bond are pulled more towards the oxygen atom, creating a partial negative charge (δ-) on the oxygen and a partial positive charge (δ+) on the carbon.
Additionally, the CO molecule has a linear geometry, with carbon in the center and oxygen on one side. Since the oxygen atom has a higher electronegativity, the polar bond moments (represented by arrows pointing towards the more electronegative oxygen atom) do not cancel each other out. Instead, they result in a net dipole moment along the carbon-oxygen bond.
ii. 
NO2 (nitrogen dioxide) is a polar molecule due to the presence of a polar bond and an asymmetrical molecular geometry. In a NO2 molecule, nitrogen (N) and oxygen (O) are bonded together by a covalent bond. Oxygen is more electronegative than nitrogen, which means it has a higher affinity for electrons. As a result, the shared electrons in the nitrogen-oxygen bond are pulled more towards the oxygen atom, creating a partial negative charge (δ-) on the oxygen and a partial positive charge (δ+) on the nitrogen.
Furthermore, the NO2 molecule has a bent or V-shaped geometry. This shape arises due to the repulsion between the lone pair of electrons on the nitrogen atom and the bonding electron pairs. The lone pair pushes the oxygen atoms closer together, resulting in an asymmetrical distribution of charge.Due to the presence of a polar bond (N-O) and the asymmetrical molecular geometry, the individual bond dipoles do not cancel each other out. Instead, they combine to give the molecule a net dipole moment.
iii.![]()
CO2 (carbon dioxide) is a nonpolar molecule due to its symmetrical linear molecular geometry and the cancellation of dipole moments.
In a CO2 molecule, carbon is bonded to two oxygen atoms through double bonds. Carbon and oxygen have different electronegativities, with oxygen being more electronegative. However, the molecule’s linear geometry ensures that the bond dipoles between carbon and each oxygen are equal in magnitude but opposite in direction.
The two oxygen atoms are positioned on opposite sides of the carbon atom, resulting in a linear arrangement. Since the dipole moments of the two carbon-oxygen bonds are equal and pointing in opposite directions, they cancel each other out. This cancellation of dipole moments leads to a net dipole moment of zero, making CO2 a nonpolar molecule.
