Question
Which statement best describes the intramolecular bonding in HCN(l)?
A. Electrostatic attractions between \({{\text{H}}^ + }\) and \({\text{C}}{{\text{N}}^ – }\) ions
B. Only van der Waals’ forces
C. Van der Waals’ forces and hydrogen bonding
D. Electrostatic attractions between pairs of electrons and positively charged nuclei
▶️Answer/Explanation
D
HCN has Electrostatic attractions between pairs of electrons of \({\text{C}}{{\text{N}}^ – }\) ion and positively charged nuclei of \({{\text{H}}^ + }\) .
Question
Which compound does not form hydrogen bonds between its molecules?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{H}}_{\text{2}}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
▶️Answer/Explanation
B
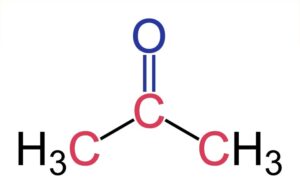
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}\) does not form hydrogen bond. For the formation of hydrogen bond, at least one hydrogen atom must be bonded to one of the three most electronegative atoms, i.e. O,N and F.
Question
Which substance can form intermolecular hydrogen bonds in the liquid state?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OC}}{{\text{H}}_{\text{3}}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
▶️Answer/Explanation
B
Out of these, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\) can form hydrogen bond. For the formation of hydrogen bond, at least one hydrogen atom must be bonded to one of the three most electronegative atoms, i.e, O,N and F.
Question
Which order is correct when the following compounds are arranged in order of increasing melting point?
A. \({\text{C}}{{\text{H}}_{\text{4}}} < {{\text{H}}_{\text{2}}}{\text{S}} < {{\text{H}}_{\text{2}}}{\text{O}}\)
B. \({{\text{H}}_{\text{2}}}{\text{S}} < {{\text{H}}_{\text{2}}}{\text{O}} < {\text{C}}{{\text{H}}_{\text{4}}}\)
C. \({\text{C}}{{\text{H}}_{\text{4}}} < {{\text{H}}_{\text{2}}}{\text{O}} < {{\text{H}}_{\text{2}}}{\text{S}}\)
D. \({{\text{H}}_{\text{2}}}{\text{S}} < {\text{C}}{{\text{H}}_{\text{4}}} < {{\text{H}}_{\text{2}}}{\text{O}}\)
▶️Answer/Explanation
A
Among these, water exhibits hydrogen bonding between adjacent molecules and melts at the highest temperature.
H2S is polar, but not H-bonded and has the second highest melting point.
The molecules of methane are non-polar, and will be held together by London dispersion forces, hence it will have minimum melting point.
Question
Which compound forms hydrogen bonds in the liquid state?
A. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}}\)
B. \({\text{CHC}}{{\text{l}}_{\text{3}}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\)
D. \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{3}}}{\text{N}}\)
▶️Answer/Explanation
A
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}}\) has an H atom directly bonded with O atom, hence it will have H bonds.
\({\text{CHC}}{{\text{l}}_{\text{3}}}\) doesn’t have any H atom which is directly bonded with one of the most electronegative atoms F,N or O. Hence, it does not form hydrogen bonding between its molecules.
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\) which is an aldehyde, and this does not form hydrogen bonding between its molecules.
Structure of \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{3}}}{\text{N}}\) is: 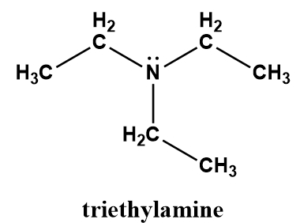
which doesn’t have an H atom directly bonded with one of the most electronegative atoms F,N or O. Hence, it does not form hydrogen bonding between its molecules.
Hence the only correct answer is A. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}}\).
Question
Which change explains why the boiling points of the halogens increase as their molecular masses increase?
A. The intermolecular attraction due to temporarily induced dipoles increases.
B. The gravitational attraction between molecules increases.
C. The polarity of the bond within the molecule increases.
D. The strength of the bond within the molecule increases.
▶️Answer/Explanation
A
The molecular mass of the halogens increases as you go down the group. The halogens exist as diatomic molecules, with both atoms sharing an electron to completely fill the outer shell. The increase in boiling point can be attributed to the increase in intermolecular forces (van der Waal forces). The number of electrons increases in each element going down the group, this leads to an increase in temporarily induced dipoles.
Question
Which correctly states the strongest intermolecular forces in the compounds below?
▶️Answer/Explanation
B
C-Cl bond is polar. Hence, CH₃Cl is a polar molecule, while CH₄ is non-polar. Therefore, the van der Waals’ forces between CH₃Cl molecules are dipole-dipole attractions, while the van der Waals’ forces between CH₄ molecules are London dispersion forces.
Since in CH₃NH2 , hydrogen is attached to electronegative nitrogen, hence, Hydrogen bonding exists in CH₃NH2.
