Question
Sodium carbonate and hydrochloric acid react according to the equation below.
\[{\text{N}}{{\text{a}}_2}{\text{C}}{{\text{O}}_3}({\text{s)}} + 2{\text{HCl(aq)}} \to {\text{C}}{{\text{O}}_2}({\text{g)}} + 2{\text{NaCl(aq)}} + {{\text{H}}_2}{\text{O(l)}}\]
Which conditions will produce the fastest initial rate with 2.0 g of powdered sodium carbonate?
A. \({\text{100 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) hydrochloric acid at 323 K
B. \({\text{50 c}}{{\text{m}}^{\text{3}}}\) of \({\text{2.0 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) hydrochloric acid at 323 K
C. \({\text{100 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) hydrochloric acid at 348 K
D. \({\text{50 c}}{{\text{m}}^{\text{3}}}\) of \({\text{2.0 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) hydrochloric acid at 348 K
▶️Answer/Explanation
D
Both 50 cm3 of 2 mol dm-3 HCl and 100 cm3 of 1 mol dm-3 HCl have equal 0.1 mol HCl.
Now, faster reaction will occur in case of 50 cm3 of 2 mol dm-3HCl as it has smaller volume within which the reaction is taking place which increases the probability of successful collisions.
Also, rate of reaction will be more at higher temperature i.e. at 348K than 323K.
Question
What is the best definition of rate of reaction?
A. The time it takes to use up all the reactants
B. The rate at which all the reactants are used up
C. The time it takes for one of the reactants to be used up
D. The increase in concentration of a product per unit time
▶️Answer/Explanation
D
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit time.
Question
The diagram below shows the energy changes for a reaction with and without a catalyst. Which symbols represent the activation energy, \({E_{\text{a}}}\), and the enthalpy change, \(\Delta H\), for the reaction with a catalyst?
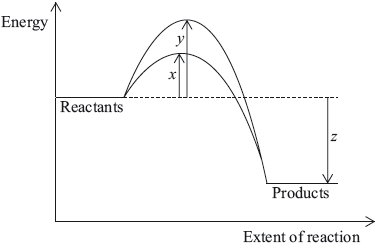
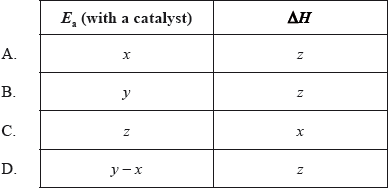
▶️Answer/Explanation
A
As catalyst lowers the activation energy barrier, hence x is the Ea with a catalyst.
Enthalpy change is the difference between energy levels of products and reactants, it is z here.
Question
Which statements explain why a catalyst is used in the Contact process (shown below)?
\[{\text{S}}{{\text{O}}_2}{\text{(g)}} + \frac{1}{2}{{\text{O}}_2}{\text{(g)}} \rightleftharpoons {\text{S}}{{\text{O}}_3}{\text{(g)}}\]
I. A catalyst creates a new reaction pathway of lower activation energy.
II. A catalyst moves the position of equilibrium towards the product.
III. A catalyst allows the same rate to be achieved at a lower temperature.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
▶️Answer/Explanation
B
A catalyst creates a new reaction pathway of lower activation energy.
A catalyst allows the same rate to be achieved at a lower temperature.
Adding a catalyst does not affect the relative rates of the two reactions, it cannot affect the position of equilibrium.
