Question
A student investigated how the type of acid in acid deposition affects limestone, a building material mainly composed of calcium carbonate.
The student monitored the mass of six similarly sized pieces of limestone. Three were placed in beakers containing $200.0 \mathrm{~cm}^3$ of $0.100 \mathrm{~mol} \mathrm{dm}^{-3}$ nitric acid, $\mathrm{HNO}_3(\mathrm{aq})$, and the other three in $200.0 \mathrm{~cm}^3$ of $0.100 \mathrm{~mol} \mathrm{dm}^{-3}$ sulfuric acid, $\mathrm{H}_2 \mathrm{SO}_4$ (aq).
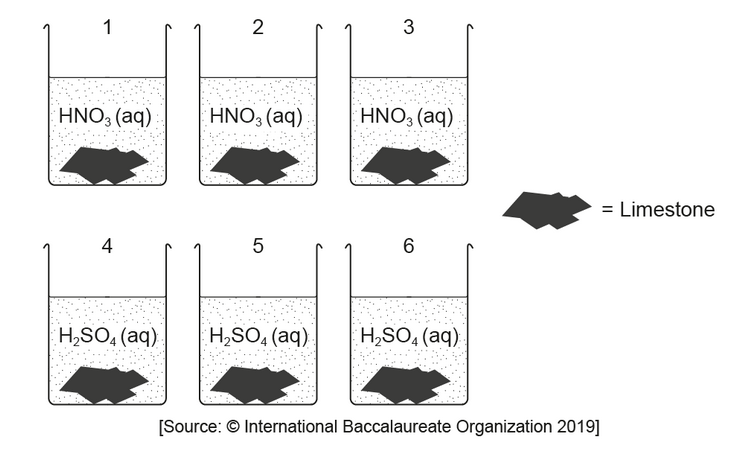
a. Draw a best-fit line on the graph.
$b(i)$ Determine the initial rate of reaction of limestone with nitric acid from the graph.
Show your working on the graph and include the units of the initial rate.
$b$ (iiExplain why the rate of reaction of limestone with nitric acid decreases and reaches zero over the period of five days.
$\mathrm{b}$ (iiisuggest a source of error in the procedure, assuming no human errors occurred and the balance was accurate.
c(i)Justify this hypothesis.
c(ii)The student obtained the following total mass losses.
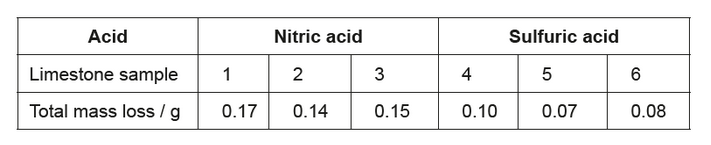
She concluded that nitric acid caused more mass loss than sulfuric acid, which did not support her hypothesis.
Suggest an explanation for the data, assuming that no errors were made by the student.
▶️Answer/Explanation
Markscheme
a. best-fit smooth curve
NOTE: Do not accept a series of connected lines that pass through all points OR any straight line representation.
b(i)tangent drawn at time zero
$\mathrm{g} \mathrm{day}^{-1}$
$0.16 \checkmark$
NOTE: Accept other reasonable units for initial rate eg, $\mathrm{mol} \mathrm{dm}^{-3} \mathrm{~s}^{-1}, \mathrm{~mol} \mathrm{dm}^{-3} \mathrm{~min}^{-1}, \mathrm{~g} \mathrm{~s}^{-1} \mathrm{OR} \mathrm{g} \mathrm{min}^{-1}$.
$M 3$ can only be awarded if the value corresponds to the correct unit given in M2.
Accept values for the initial rate for M3 in the range: $0.13-0.20 \mathrm{~g} \mathrm{day}^{-1}$ OR $1.5 \times 10^{-6} \mathrm{~g} \mathrm{~s}^{-1}-2.3 \times 10^{-6} \mathrm{~g} \mathrm{~s}^{-1}$ OR $7.5 \times 10^{-8}-1.2 \times 10^{-7} \mathrm{~mol} \mathrm{dm}^{-3}$ $\mathrm{s}^{-1}$ OR $4.5 \times 10^{-6}-6.9 \times 10^{-6} \mathrm{~mol} \mathrm{dm}^{-3} \mathrm{~min}^{-1}$ OR $9.0 \times 10^{-5}-1.4 \times 10^{-4} \mathrm{~g} \mathrm{~min}^{-1}$ OR a range based on any other reasonable unit for rate.
Ignore any negative rate value.
Award [ 2 max] for answers such as $0.12 / 0.11 \mathrm{~g} \mathrm{day}^{-1}$, incorrectly obtained by using the first two points on the graph (the average rate between $t=0$ and 1 day).
Award [1 max] for correctly calculating any other average rate.
OR
acid is the limiting reactant
concentration of acid decreases
OR
less frequent collisions
NOTE: Award [1 max] for “surface area decreases” if the idea that $\mathrm{CaCO}_3$ is used up/acts as the limiting reactant” is conveyed for M1.
Do not accept “reaction reaches equilibrium” for M2.
$b$ (iiisurface area not uniform
NOTE: Accept “acids impure.
OR
limestone pieces do not have same composition/source
NOTE: Accept “«limestone» contains impurities”.
OR
limestone absorbed water «which increased mass»
OR
acid removed from solution when limestone removed
NOTE: Accept “loss of limestone when dried” OR “loss of limestone due to crumbling when removed from beaker”.
OR
“some» calcium sulfate deposited on limestone lost
OR
pieces of paper towel may have stuck to limestone
beakers not covered/evaporation
OR
temperature was not controlled
c(i) sulfuric acid is diprotic/contains two $\mathrm{H}^{+}$«while nitric acid contains one $\mathrm{H}^{+}$)/releases more $\mathrm{H}^{+}$«so reacts with more limestone»
OR
higher concentration of protons/ $/ \mathrm{H}^{+} \checkmark$
NOTE: Ignore any reference to the relative strengths of sulfuric acid and nitric acid.
Accept “sulfuric acid has two hydrogens “whereas nitric has one»”.
Accept “dibasic” for “diprotic”.
c(ii)calcium sulfate remained/deposited on limestone «in sulfuric acid»
OR
reaction prevented/stopped by slightly soluble/deposited/layer of calcium sulfate
Question
Antacids react with hydrochloric acid in the stomach to relieve indigestion. A student investigated different brands of antacid to see which caused the largest increase in pH in a given time. She added the antacids to hydrochloric acid, and recorded the change in pH over five minutes.
State an equation for the reaction of magnesium hydroxide with hydrochloric acid.
Suggest two variables, besides the time of reaction, which the student should have controlled in the experiment to ensure a fair comparison of the antacids.
Calculate the uncertainty in the change in pH.
The student concluded that antacid B was the most effective, followed by A then C and finally D. Discuss two arguments that reduce the validity of the conclusion.
▶️Answer/Explanation
Markscheme
Mg(OH)2 (s) + 2HCl (aq) → MgCl2 (aq) + 2H2O (l)
Accept full or net ionic equation.
Any two from:
volume «of HCl»
concentration «of HCl»/[HCl]
temperature «of HCl»
mass of antacid/tablets
size of antacid particles/tablets
OR
surface area of antacid «particles»/tablets
Accept “number of tablets/different doses”.
Do not accept “same pH meter” OR “initial pH” OR “concentration of antacid/[antacid]”.
A variable must be given so do not accept answers such as “stirring”, “whether tablets are whole or crushed” etc.
[Max 2 Marks]
(±) 0.04
OR
(±) 0.03
Any two of:
uncertainty «(±)0.04/(±)0.03» means A and C cannot be distinguished
each measurement was conducted once
stomach pH should not be raised a lot «so antacid B is not necessarily effective»
mass/number of tablets/dose «of antacid» used was not controlled
actual environment in stomach is different
Accept “amount of tablets” for “dose”.
Do not accept “nature/composition of tablets differs”.
Accept an answer such as “time frame is too short since some antacids could be long-acting drugs if they contain a gelatinisation/delaying agent” but not just “time frame is too short since some antacids could be long-acting drugs”.
[Max 2 Marks]
Question
Palmitic acid has a molar mass of 256.5 g mol−1.
The apparatus in the diagram measures the surface pressure created by palmitic acid molecules on the surface of water. This pressure is caused by palmitic acid molecules colliding with the fixed barrier. The pressure increases as the area, A, available to the palmitic acid is reduced by the movable barrier.
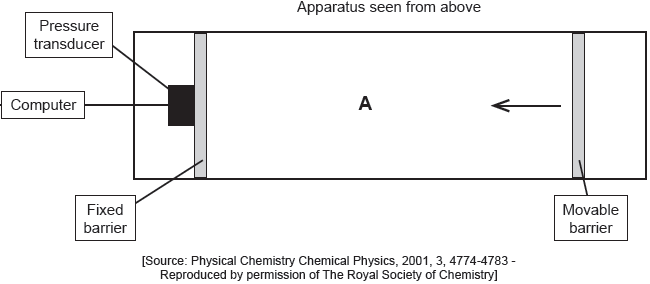
When a drop of a solution of palmitic acid in a volatile solvent is placed between the barriers, the solvent evaporates leaving a surface layer. The graph of pressure against area was obtained as the area A was reduced.
Part of this molecule is hydrophilic (bonds readily to water) and part hydrophobic (does not bond readily to water). Draw a circle around all of the hydrophilic part of the molecule.
When a small amount of palmitic acid is placed in water it disperses to form a layer on the surface that is only one molecule thick. Explain, in terms of intermolecular forces, why this occurs.
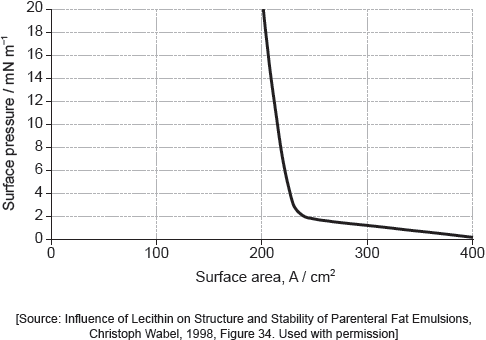
Suggest why there is a small increase in the surface pressure as the area is reduced to about 240 cm2, but a much faster increase when it is further reduced.
The solution of palmitic acid had a concentration of 0.0034 mol dm−3. Calculate the number of molecules of palmitic acid present in the 0.050 cm3 drop, using section 2 of the data booklet.
Assuming the sudden change in gradient occurs at 240 cm2, calculate the area, in cm2, that a single molecule of palmitic acid occupies on surface of the water.
If you did not obtain an answer for (b)(ii) use a value of 8.2 × 1016, but this is not the correct answer.
▶️Answer/Explanation
Markscheme
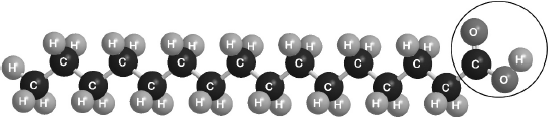
Must cut CH2–CO bond AND enclose all of the –COOH group.
[1 mark]
Any two of:
–COOH/CO/OH/carboxylate/carboxyl/hydroxyl/hydroxy group forms hydrogen bonds/H-bonds to water
London/dispersion/instantaneous induced dipole-induced dipole forces occur between hydrocarbon chains
hydrocarbon chain cannot form hydrogen bonds/H-bonds to water
strong hydrogen bonds/H-bonds between water molecules exclude hydrocarbon chains «from the body of the water»
Accept “hydrophilic part/group forms hydrogen bonds/H-bonds to water”.
Accept “hydrophobic section” instead of “hydrocarbon chain”.
Award [1 max] for answers based on “the –COOH group being polar AND the hydrocarbon chain being non-polar”.
[2 marks]
Above about 240 cm2:
greater collision frequency/collisions per second between «palmitic acid» molecules and the barrier «as area reduced»
At less than about 240 cm2:
molecules completely cover the surface
OR
there is no space between molecules
OR
force from movable barrier transmitted directly through the molecules to the fixed barrier
OR
«palmitic acid» molecules are pushed up/down/out of layer
For both M1 and M2 accept “particles” for “molecules”.
For M1 accept “space/area between molecules reduced” OR “molecules moving closer together”.
[2 marks]
amount of acid = «5.0 × 10–5 dm3 × 0.0034 mol dm–3» = 1.7 × 10–7 «mol»
number of molecules = «1.7 × 10–7 mol × 6.02 × 1023 mol–1 =» 1.0 × 1017
Award [2] for correct final answer.
Award [1] for “1.0 × 1020”.
[2 marks]
«area = \(\frac{{240{\text{ c}}{{\text{m}}^2}}}{{1.0 \times {{10}^{17}}}}\) » 2.4 × 10–15 «cm2»
[1 mark]
Question
Students were asked to investigate how a change in concentration of hydrochloric acid, HCl, affects the initial rate of its reaction with marble chips, CaCO3.
They decided to measure how long the reaction took to complete when similar chips were added to 50.0 cm3 of 1.00 mol dm−3 acid and 50.0 cm3 of 2.00 mol dm−3 acid.
Two methods were proposed:
(1) using small chips, keeping the acid in excess, and recording the time taken for the solid to disappear
(2) using large chips, keeping the marble in excess, and recording the time taken for bubbles to stop forming.
A group recorded the following results with 1.00 mol dm−3 hydrochloric acid:
Annotate the balanced equation below with state symbols.
CaCO3(__) + 2HCl(__) → CaCl2(__) + CO2(__) + H2O(__)
Neither method actually gives the initial rate. Outline a method that would allow the initial rate to be determined.
Deduce, giving a reason, which of the two methods would be least affected by the chips not having exactly the same mass when used with the different concentrations of acid.
State a factor, that has a significant effect on reaction rate, which could vary between marble chips of exactly the same mass.
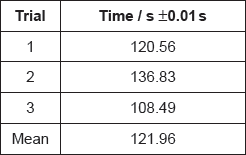
Justify why it is inappropriate to record the uncertainty of the mean as ±0.01 s.
If doubling the concentration doubles the reaction rate, suggest the mean time you would expect for the reaction with 2.00 mol dm−3 hydrochloric acid.
Another student, working alone, always dropped the marble chips into the acid and then picked up the stopwatch to start it. State, giving a reason, whether this introduced a random or systematic error.
▶️Answer/Explanation
Markscheme
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
Accept “CO2(aq)”.
[1 mark]
measure the volume of gas at different times «plot a graph and extrapolate»
OR
measure the mass of the reaction mixture at different times «plot a graph and extrapolate»
Accept other techniques that yield data which can be plotted and extrapolated.
[1 mark]
method 2 AND marble is in excess «so a little extra has little effect»
OR
large chips AND marble is in excess «so a little extra has little effect»
OR
method 2 AND HCl is limiting reagent «so a little extra marble has little effect»
OR
large chips AND HCl is limiting reagent «so a little extra marble has little effect»
Accept, as a reason, that “as the mass is greater the percentage variation will be lower”.
[1 mark]
surface area
OR
purity «of the marble»
Accept “shape of the chip”.
[1 mark]
variation of individual values is much greater «than this uncertainty»
OR
«uncertainty» does not take into account «student» reaction time
[1 mark]
«\(\frac{{121.96{\text{ s}}}}{2}\) = 60.98 s» = 61 «s»
[1 mark]
systematic AND always makes the time shorter «than the actual value»
OR
systematic AND it is an error in the method used «not an individual measurement»
OR
systematic AND more repetitions would not reduce the error
Accept, as reason, “it always affects the value in the same direction” OR “the error is consistent”.
[1 mark]
Question
Penicillins and aspirin are important medicines.
Describe how penicillin combats bacterial infections.
State how penicillins may be modified to increase their effectiveness.
State the type of reaction used to synthesize aspirin from salicylic acid.
Explain why aspirin is not stored in a hot, humid location.
▶️Answer/Explanation
Markscheme
«irreversibly» binds/bonds to enzyme/transpeptidase
OR
inhibits enzyme/transpeptidase «in bacteria» that produces cell walls
OR
prevents cross-linking of bacterial cell walls
cells absorb water AND burst
OR
cells cannot reproduce
[2 marks]
modify side chain
[1 mark]
condensation
OR
esterification
OR
nucleophilic substitution/nucleophilic displacement/SN2
Do not accept just “substitution/displacement”.
[1 mark]
water causes hydrolysis
OR
aspirin reacts with water
heat increases the rate of hydrolysis
OR
heat increases the rate of the reaction with water
Accept “aspirin will convert into salicylic/ethanoic acid”.
Do not accept “aspirin dissolves in water” OR “aspirin absorbs water/is hygroscopic”
[2 marks]

