Question
Which are definitions of an acid according to the Brønsted-Lowry and Lewis theories?
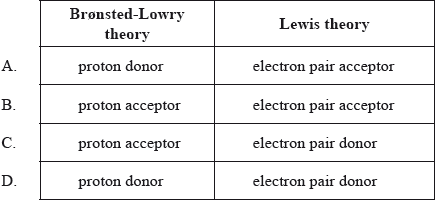
▶️Answer/Explanation
A
According to Bronsted-Lowry theory, acid is a substance which donates an H+ ion or a proton and forms its conjugate base and the base is a substance which accepts an H+ ion or a proton and forms its conjugate acid.
Lewis Acid: A species that accepts an electron pair (i.e., an electrophile) and will have vacant orbitals.
Lewis Base: a species that donates an electron pair (i.e., a nucleophile) and will have lone-pair electrons.
Question
What is the formula of the conjugate base of the hydrogen phosphate ion, \({\rm{HPO}}_4^{2 – }\)?
A. \({{\text{H}}_{\text{2}}}{\text{PO}}_4^ – \)
B. \({{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\)
C. \({\text{HPO}}_4^ – \)
D. \({\text{PO}}_4^{3 – }\)
▶️Answer/Explanation
D
An acid donates proton and conjugate base is formed.
HPO42−→H++PO43−
Question
Which species behave as Brønsted-Lowry acids in the following reversible reaction?
\[{{\text{H}}_2}{\text{PO}}_4^ – ({\text{aq)}} + {\text{C}}{{\text{N}}^ – }({\text{aq)}} \rightleftharpoons {\text{HCN(aq)}} + {\text{HPO}}_4^{2 – }({\text{aq)}}\]
A. HCN and \({\text{C}}{{\text{N}}^ – }\)
B. HCN and \({\text{HPO}}_4^{2 – }\)
C. \({{\text{H}}_{\text{2}}}{\text{PO}}_4^ – \) and \({\text{HPO}}_4^{2 – }\)
D. HCN and \({{\text{H}}_{\text{2}}}{\text{PO}}_4^ – \)
▶️Answer/Explanation
D
Both HCN and \({{\text{H}}_{\text{2}}}{\text{PO}}_4^ – \) are donating proton H+ here, hence these are Brønsted-Lowry acids in the reversible reaction.
Question
What is the conjugate base of \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) according to the Brønsted-Lowry theory?
A. \({\text{CO}}_{\text{3}}^{{\text{2}} – }\)
B. \({\text{HCO}}_{\text{3}}^ – \)
C. \({{\text{H}}_{\text{3}}}{\text{CO}}_{\text{3}}^ + \)
D. \({\text{C}}{{\text{O}}_{\text{2}}}\)
▶️Answer/Explanation
B
An acid donates H+ to form its conjugate base. Thus, conjugate base of a compound has one H+ less. Hence conjugate base of H2CO3 is HCO3−.
Question
Consider the equilibrium below.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CO}}{{\text{O}}^ – }{\text{(aq)}} + {{\text{H}}_{\text{3}}}{{\text{O}}^ + }{\text{(aq)}}\]
Which species represent a conjugate acid-base pair?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\) and \({{\text{H}}_{\text{2}}}{\text{O}}\)
B. \({{\text{H}}_{\text{2}}}{\text{O}}\) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CO}}{{\text{O}}^ – }\)
C. \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) and \({{\text{H}}_{\text{2}}}{\text{O}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CO}}{{\text{O}}^ – }\) and \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\)
▶️Answer/Explanation
C
An acid donates H+ to form its conjugate base. Thus, conjugate base of an acid has one H+ less. Here, acid is \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) and its conjugate base is \({{\text{H}}_{\text{2}}}{\text{O}}\) .
