Question
Which solution has a pH of 9?
A 1000 × 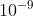 mol dm-3 HCl (aq)
mol dm-3 HCl (aq)
B 1.0 × 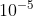 mol dm-3 KOH (aq)
mol dm-3 KOH (aq)
C 1.0 × 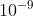 mol dm-3 KOH (aq)
mol dm-3 KOH (aq)
D 1.0 × 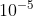 mol dm-3 HCl (aq)
mol dm-3 HCl (aq)
▶️Answer/Explanation
Ans: B
pH = -Log[H+]
pOH=-Log[OH–]
pH + pOH = 14
In B, pOH = 5
Therefore, pH =9.
Question
What is the correct expression for the ionic product constant of water, \({K_{\text{w}}}\)?
A. \({K_{\text{W}}} = \frac{{{\text{[}}{{\text{H}}^ + }{\text{]}}}}{{{\text{[O}}{{\text{H}}^ – }{\text{]}}}}\)
B. \({K_{\text{W}}}{\text{ = }}\frac{{{\text{[}}{{\text{H}}_2}{\text{O]}}}}{{{\text{[}}{{\text{H}}^ + }{\text{][O}}{{\text{H}}^ – }{\text{]}}}}\)
C. \({K_{\text{W}}} = {\text{[}}{{\text{H}}^ + }{\text{]}} + {\text{[O}}{{\text{H}}^ – }{\text{]}}\)
D. \({K_{\text{W}}} = {\text{[}}{{\text{H}}^ + }{\text{][O}}{{\text{H}}^ – }{\text{]}}\)
▶️Answer/Explanation
D
Ionic product constant of water, \({K_{\text{W}}} = {\text{[}}{{\text{H}}^ + }{\text{][O}}{{\text{H}}^ – }{\text{]}}\).
Question
What is the pH of 1.0 × 10−3 mol dm−3 sodium hydroxide, NaOH(aq)?
Kw = 1.0 × 10−14
A. 3
B. 4
C. 10
D. 11
▶️Answer/Explanation
D
pH + pOH = 14
1 dm−3 = 1 ltr
Here, pOH = -Log[OH–] = 3
pH =11.
Question
What is the pH of a solution in which the hydroxide ion concentration is 1 × 10−11 mol dm−3 at 298 K?
Kw = 1 × 10−14 at 298 K
A. 3
B. 7
C. 11
D. 14
▶️Answer/Explanation
A
pH + pOH = 14
1 dm−3 = 1 ltr
Here, pOH = -Log[OH–] = 11
pH =3.
