Question
Chlorine gas reacts with water to produce hypochlorous acid and hydrochloric acid.
Cl2 (g) + H2O (l) HClO (aq) + HCl (aq)
(i) Hypochlorous acid is considered a weak acid. Outline what is meant by the term weak acid. [1]
(ii) State the formula of the conjugate base of hypochlorous acid. [1]
(iii) Calculate the concentration of H+ (aq) in a HClO (aq) solution with a pH = 3.61. [1]
Answer/Explanation
Ans
i partially dissociates/ionizes «in water»
ii ClO–
iii «[H+] = 10−3.61 =» 2.5 × 10−4 «mol dm−3»
Question
Some of the most important processes in chemistry involve acid-base reactions.
Describe the acid-base character of the oxides of each of the period 3 elements, Na to Cl.
State one example of an acidic gas, produced by an industrial process or the internal combustion engine, which can cause large-scale pollution to lakes and forests.
Suggest one method, other than measuring pH, which could be used to distinguish between solutions of a strong acid and a weak acid of the same molar concentration. State the expected results.
Answer/Explanation
Markscheme
Na, Mg: basic;
Al: amphoteric;
Do not accept amphiprotic.
Si to Cl: acidic;
Award [1] for stating oxides become more basic towards left/Na and more acidic towards right/Cl.
Do not penalize incorrect formulas of oxides.
\({\text{N}}{{\text{O}}_{\text{2}}}\)/nitrogen dioxide / \({{\text{N}}_{\text{2}}}{{\text{O}}_{\text{4}}}\)/dinitrogen tetroxide / \({\text{S}}{{\text{O}}_{\text{2}}}\)/sulfur dioxide / \({\text{S}}{{\text{O}}_{\text{3}}}\)/sulfur trioxide;
Do not accept NO/NOx/CO2/CO.
measure electrical conductivity;
strong acids are good conductors/better conductors than weak acids / weak acids are poor conductors;
OR
react with magnesium or a named active metal/metal carbonate/hydrogen carbonate/bicarbonate;
Do not accept Na/K
strong acids react faster/more gas bubbles (per unit time)/more heat produced / weak acids react slower/less gas bubbles (per unit time)/less heat produced;
Do not accept answers based on titration curves as they are based on pH.
Accept Neutralization: weak acid would produce less energy/less temperature increase compared to a strong acid.
Examiners report
The majority of candidates gave the correct answers to (a), but a few were confused about the acid-base character of the oxides of aluminium and silicon.
Part (b) proved to be a difficult question. Not many candidates gave the name or formula of an acidic gas produced by an industrial process. Some wrong answers were: CO, SO, \({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\), CFCs, Methane, \({\text{N}}{{\text{H}}_{\text{3}}}\).
There were a few good answers to (c); measuring the conductivity or the reaction with magnesium or calcium carbonate was a possible method for distinguishing between a strong and a weak acid of the same concentration.
Question
Water is an important substance that is abundant on the Earth’s surface. Water dissociates according to the following equation.
\[{{\text{H}}_{\text{2}}}{\text{O(l)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{O}}{{\text{H}}^ – }{\text{(aq)}}\]
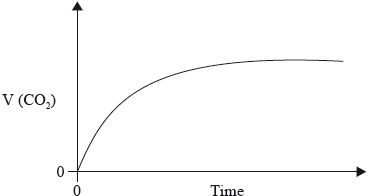
The graph below shows how the volume of carbon dioxide formed varies with time when a hydrochloric acid solution is added to excess calcium carbonate in a flask.
(i) State the equilibrium constant expression for the dissociation of water.
(ii) Explain why even a very acidic aqueous solution still has some \({\text{O}}{{\text{H}}^ – }\) ions present in it.
(iii) State and explain the effect of increasing temperature on the equilibrium constant above given that the dissociation of water is an endothermic process.
(iv) The pH of a solution is 2. If its pH is increased to 6, deduce how the hydrogen ion concentration changes.
In carbonated drinks containing dissolved carbon dioxide under high pressure, the
following dynamic equilibrium exists.
\[{\text{C}}{{\text{O}}_2}({\text{aq)}} \rightleftharpoons {\text{C}}{{\text{O}}_2}({\text{g)}}\]
Describe the effect of opening a carbonated drink container and outline how this
equilibrium is affected.
(i) Explain the shape of the curve.
(ii) Copy the above graph on your answer sheet and sketch the curve you would obtain if double the volume of hydrochloric acid solution of half the concentration as in the example above is used instead, with all other variables kept constant from the original. Explain why the shape of the curve is different.
(iii) Outline one other way in which the rate of this reaction can be studied in a school laboratory. Sketch a graph to illustrate how the selected variable would change with time.
(iv) Define the term activation energy and state one reason why the reaction between calcium carbonate and hydrochloric acid takes place at a reasonably fast rate at room temperature.
Answer/Explanation
Markscheme
(i) \({K_{\text{c}}} = \frac{{{\text{[}}{{\text{H}}^ + }{\text{][O}}{{\text{H}}^ – }{\text{]}}}}{{{\text{[}}{{\text{H}}_2}{\text{O]}}}}/{K_{\text{c}}} = \frac{{{{{\text{[}}{{\text{H}}_3}{\text{O]}}}^ + }{\text{[O}}{{\text{H}}^ – }{\text{]}}}}{{{\text{[}}{{\text{H}}_2}{\text{O]}}}}/{K_{\text{w}}} = {\text{[}}{{\text{H}}^ + }{\text{][O}}{{\text{H}}^ – }{\text{]}}/{K_{\text{w}}} = {\text{[}}{{\text{H}}_{\text{3}}}{{\text{O}}^ + }{\text{][O}}{{\text{H}}^ – }{\text{]}}\);
Do not award mark if [ ] are omitted or other brackets are used.
Expression must be consistent with \({K_{\text{c}}}/{K_{\text{w}}}\).
(ii) \({\text{[}}{{\text{H}}^ + }{\text{]}}\) increases, \({\text{[O}}{{\text{H}}^ – }{\text{]}}\) decreases but still some present (\({K_{\text{w}}}/{K_{\text{c}}}\) constant) / \({\text{[O}}{{\text{H}}^ – }{\text{]}}\)
cannot go to zero as equilibrium present / \({\text{[O}}{{\text{H}}^ – }{\text{]}} = \frac{{{K_{\text{w}}}}}{{{\text{[}}{{\text{H}}^ + }{\text{]}}}}/\frac{{{K_{\text{c}}}{\text{[}}{{\text{H}}_2}{\text{O]}}}}{{{\text{[}}{{\text{H}}^ + }{\text{]}}}}\), thus \({\text{[O}}{{\text{H}}^ – }{\text{]}}\)
cannot be zero / OWTTE;
Accept equilibrium present.
(iii) (changing T disturbs equilibrium) forward reaction favoured / equilibrium shifts to the right;
to use up (some of the) heat supplied;
(\({K_{\text{w}}}/{K_{\text{c}}}\)) increases (as both \({\text{[}}{{\text{H}}^ + }{\text{]}}\) and \({\text{[O}}{{\text{H}}^ – }{\text{]}}\) increase);
(iv) \({\text{pH}} = 2{\text{, [}}{{\text{H}}^ + }{\text{]}} = 0.01{\text{ mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) and \({\text{pH}} = 6{\text{, [}}{{\text{H}}^ + }{\text{]}} = {10^{ – 6}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ – 3}}/{\text{[}}{{\text{H}}^ + }{\text{]}} = {10^{ – {\text{pH}}}}\);
\({\text{[}}{{\text{H}}^ + }{\text{]}}\) decreased/changed by \({\text{10000/1}}{{\text{0}}^{ – 4}}\);
Award [2] for correct final answer.
\({\text{C}}{{\text{O}}_2}({\text{g)}}\) /gas escapes / (gas) pressure / \({\text{C}}{{\text{O}}_2}\) (above liquid) decreases / bubbles (of \({\text{C}}{{\text{O}}_2}\) gas) form in the liquid;
equilibrium shifts to the right (to replace the lost \({\text{C}}{{\text{O}}_2}\) gas);
(i) rate = increase in \(\frac{{{\text{volume}}}}{{{\text{time}}}}\) = slope of graph;
initially/to begin with steeper slope / fastest rate / volume of gas/ \({\text{C}}{{\text{O}}_2}\) produced faster/quickly as concentration of HCl highest / OWTTE;
as reaction progresses/with time, less steep slope / volume of gas production slows / rate decreases due to less frequent collisions as concentration (of HCl) decreases / OWTTE;
curve flattens/becomes horizontal when HCl used up/consumed (as there are no more \({{\text{H}}^ + }\) ions to collide with the \({\text{CaC}}{{\text{O}}_3}\) particles);
Each mark requires explanation.
(ii) 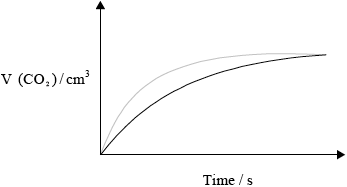
less steep curve;
same maximum volume at later time;
half/lower \({{\text{H}}^ + }\)/acid concentration less frequent collisions slower rate;
same amount of HCl, same volume \({\text{C}}{{\text{O}}_2}\) produced;
(iii) mass loss/of \({\text{C}}{{\text{O}}_2}\) / mass of flask + content;
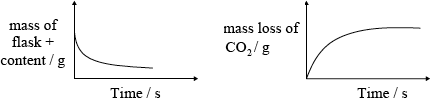 ;
;
OR
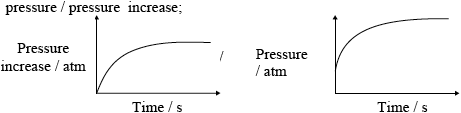 ;
;
OR
 ;
;
Do not penalize for missing x-axis label or for missing units on y-axis.
Accept if line meets time axis.
Award [1 max] if temperature is on the vertical axis and magnitude of slope decreases with time.
(iv) minimum/least energy (of colliding particles) for a reaction to occur / OWTTE;
low/lower \({E_{\text{a}}}\) /activation energy / greater/larger surface area/contact between \({\text{CaC}}{{\text{O}}_{\text{3}}}\) and HCl / high/higher HCl concentration/[HCl] / (sufficient) particles/molecules have activation energy;
Examiners report
This was the most popular question in Section B but responses were mixed. Part (a) was generally well dealt with but some candidates confused \({K_{\text{w}}}\) with \({K_{\text{c}}}\) or forgot to include charges on the ions in the equilibrium constant expression. Few received the mark for question (ii) although some mentioned equilibrium which was sufficient.
Candidates recognised that increasing the temperature shifts the equilibrium to the right, but most did not explain why, namely to use up some of the heat supplied. The calculation in (iv) was quite well done although some only gave a qualitative answer.
The equilibrium of carbonated drinks was well understood.
In part (c) (i) candidates frequently described the shape of the curve instead of offering an explanation using collisions theory. Candidates did state, for example, that the curve flattens but did not refer to consumption of HCl(aq), the limiting reagent. Only the better candidates were only able to link slope with rate and some still consider the rate to increase after the reaction has started. In (ii) most realised that the curve would be less steep but few drew a curve with the same maximum volume produced at a later time. Even fewer candidates were able to explain why the number of moles of carbon dioxide remained the same. Although some candidates chose mass loss / pH / pressure as the dependant variable in c(iii), some were penalised for imprecise answers such as mass of reactants without referring to mass of flask. Others misunderstood the question and described experiments that they had done with catalysis or described changes with temperature as the dependant variable. (c)(iv) was generally well answered, but again some responses lacked precision; the activation energy is the minimum energy needed for a reaction to occur.