Question
Identify the type of bond and by-product when monosaccharides combine.
Bond:
By-product:
Answer/Explanation
Answer:
Bond:
glycosidic
By-product:
water/\(H_2O \)
Question
Lipids are another group of biomolecules.
(a) Compare the hydrolytic and oxidative rancidity and contrast the site where the chemical changes occur.
Compare rancidity:
Contrast reaction site:
(b) Calculate the iodine number for ozubondo acid, \(C_{21}H_{33}COOH\).
\(M_r\) = 330.56
(c) Explain two ways in which carbohydrates and lipids differ as sources of energy.
Answer/Explanation
Answer:
(a) Compare rancidity:
«both produce» disagreeable smell/taste/texture/appearance
Contrast reaction site:
hydrolytic reaction occurs at ester link/COOC link AND oxidative reaction occurs
at carbon-carbon double bond/C=C
(b) 5 C=C
«100 g/330.56 g \(mol^{-1}\) x 5 x 253.8 g \(mol^{-1}\) =» 383.89 «g \(I_2\)»
(c) lipids are more reduced AND release/store more energy than carbohydrates «per gram»
lipids are less «water» soluble AND energy is released slower/less available than
in carbohydrates
Question
Lipids and carbohydrates play an essential role in the body.
(a) (i) State the general formula of a carbohydrate.
(ii) Fructose is a carbohydrate. Determine the energy, in kJ, released by the respiration of 10.5g of fructose, \(C_6H_{12}O_6\).
Enthalpy of (\(ΔH^{\theta }_c\)) of fructose = -2810kJ \(mol^{-1}\)
(b) Glucose, an isomer of fructose, exists as two isomeric ring forms. Annotate the diagram below to complete the structure of β-glucose. Use section 34 of the data booklet.
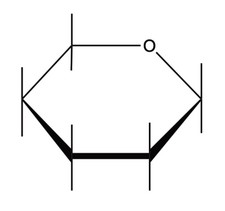
(c) Cellulose is a polymer of β-glucose. Describe the importance of cellulose in the human diet.
(d) Lipids and carbohydrates both release energy in the body.
(i) Write balanced equations for the complete oxidation of fructose, \(C_6H_{12}O_6\), and the triglyceride tristearin, \(C_{57}H_{110}O_6\).
\(C_6H_{12}O_6\):
\(C_{57}H_{110}O_6\):
(ii) Predict, giving one reason, whether 10.5g of the triglyceride tristearin would release more or less energy than 10.5g of fructose when completely oxidized.
(e) Describe the chemical composition of phospholipids and their function in the body, other than as energy storage.
Chemical composition:
Function:
Answer/Explanation
Answer:
(a) (i) \(C_x(H_2O)_y\)
(ii) «n=10.5g/180.18g \(mol^{-1}\)=» 0.0583 «mol»
«0.0583 mol x 2810 kJ \(mol^{-1}\) =» 164 «kJ»
(b) 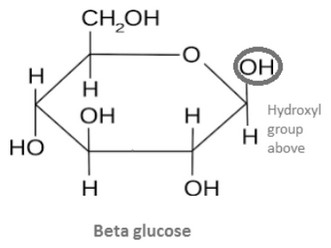
(c) «provides dietary» fibre/roughage
(d) (i) 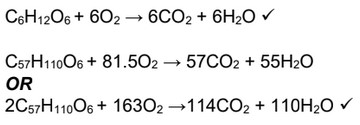
(ii) more AND contains less oxygen/contains more carbon/more reduced
(e) Chemical composition:
glycerol AND «two» fatty acids AND «one» phosphate
Function:
cell/plasma membrane