Question
A solid piece of chocolate of mass \(82 \mathrm{~g}\) is placed in a pan over fire. Thermal energy is transferred to the chocolate at a constant rate. The graph shows the variation with time \(t\), of the temperature \(T\) of the chocolate. At 6.0 minutes all the chocolate has melted.
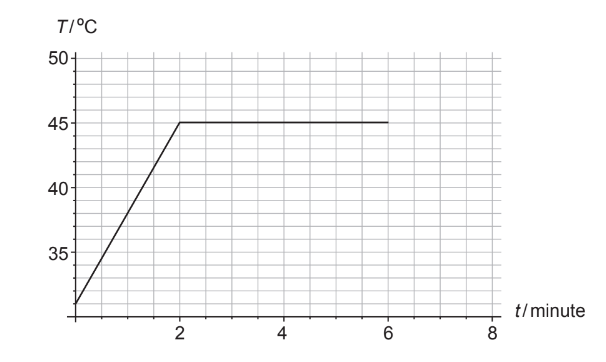
The specific heat capacity of solid chocolate is \(1.6 \times 10^3 \mathrm{~J} \mathrm{~kg}^{-1} \mathrm{~K}^{-1}\).
(a) Show that the average rate at which thermal energy is transferred into the chocolate is about \(15 \mathrm{~W}\).[3]
(b) Estimate the specific latent heat of fusion of chocolate.[2]
(c) Compare the internal energy of the chocolate at t = 2 minutes with that at t = 6 minutes. [2]
▶️Answer/Explanation
Ans:
Reads change in temperature to be \(45-31\) OR 14 ‘ \(\mathrm{C} V\)
$
Q=0.082 \times 1.6 \times 10^3 \times 14=1.84 \times 10^3 \alpha \mathrm{J} n
$
$
P=\frac{1.84 \times 10^3}{2.0 \times 60}=15.3 \approx 15 \ll W_n
$
b.\(\begin{aligned} & Q=15.3 \times 4.0 \times 60=3.67 \times 10^3 \ll \mathrm{J} » \\ & L=\frac{3.67 \times 10^3}{0.082}=4.5 \times 10^4 \alpha \mathrm{J} \mathrm{kg}^{-1} »\end{aligned}\)
c.Internal energy is greater at \(t=6 \mathrm{~min} O R\) internal energy is lower at \(t=2 \mathrm{~min} O R\) internal energy increases «as energy is added to the system»
Because kinetic energy «of the molecules» is the same AND potential energy w of the molecules was increased / OWTTE
Part 2 Internal energy
Humans generate internal energy when moving, while their core temperature remains approximately constant.
a
Distinguish between the concepts of internal energy and temperature.
An athlete loses 1.8 kg of water from her body through sweating during a training session that lasts one hour.
Estimate the rate of energy loss by the athlete due to sweating. The specific latent heat of evaporation of water is 2.3×106 J kg–1.
Answer/Explanation
Markscheme
a
internal energy:
total energy of component particles (in the human);
comprises potential energy + (random) kinetic energy;
temperature:
measure of average kinetic energy of particles;
indicates direction of (natural) flow of thermal energy;
internal energy measured in J and temperature measured
in K/°C ; (both needed)
(accept alternative suitable units)
total energy lost=2.3×106×1.8(=4.14×106J);
1.2 kW;
In an experiment to determine the specific latent heat of fusion of ice, an ice cube is dropped into water that is contained in a well-insulated calorimeter of negligible specific heat capacity. The following data are available.
Mass of ice cube = 25g
Mass of water = 350g
Initial temperature of ice cube = 0˚C
Initial temperature of water = 18˚C
Final temperature of water = 12˚C
Specific heat capacity of water = 4200Jkg−1K−1
a
Using the data, estimate the specific latent heat of fusion of ice.
The experiment is repeated using the same mass of crushed ice.
Suggest the effect, if any, of crushing the ice on
(i) the final temperature of the water.
(ii) the time it takes the water to reach its final temperature.
Answer/Explanation
Markscheme
a
use of m×c×θ with correct substitution for either original water or water from melted ice
energy available to melt ice = «8820 – 1260 =» 7560 J
equates 7560 to mL
3.02×105Jkg–1
FOR EXAMPLE
0.35 × 4200 × (18 – 12) OR 0.025 × 4200 × 12
7560 J
L=\(\frac{7560}{0.025}\)
3.02×105Jkg–1
Award [3 max] if energy to warm melted ice as water is ignored (350kJkg–1).
Allow ECF in MP3.
(i)
no change in temperature/no effect, the energies exchanged are the same
(ii)
the time will be less/ice melts faster, because surface area is greater or crushed ice has more contact with water