The mass defect for deuterium is 4×10–30 kg. What is the binding energy of deuterium?
A. 4×10–7 eV
B. 8×10–2 eV
C. 2×106 eV
D. 2×1012 eV
Answer/Explanation
Markscheme
C
\(1 kg = 6.022\times 10^{-26}amu\)
so in \(4\times 10^{-30}kg=24.088\times 10^{-4}\)
\(E_b=\Delta m.c^2\)
\(\Delta m=0.0024 u\)
\(E_b=(0.0024)\times (\frac{931MEV}{c^2})\times c^2\)
\(=2.3\times 10^6 ev\)
The nuclear reaction \({}_1^2{\rm{H}} + {}_1^3{\rm{H}} \to {}_2^4{\rm{He + }}{}_0^1{\rm{n}}\) would best be described as
A. alpha decay.
B. nuclear fission.
C. nuclear fusion.
D. neutron capture.
Answer/Explanation
Markscheme
C
Fusion reactions constitute the fundamental energy source of stars, including the Sun. The evolution of stars can be viewed as a passage through various stages as thermonuclear reactions and nucleosynthesis cause compositional changes over long time spans. Hydrogen (H) “burning” initiates the fusion energy source of stars and leads to the formation of helium (He). Generation of fusion energy for practical use also relies on fusion reactions between the lightest elements that burn to form helium. In fact, the heavy isotopes of hydrogen—deuterium (D) and tritium (T)—react more efficiently with each other, and, when they do undergo fusion, they yield more energy per reaction than do two hydrogen nuclei. (The hydrogen nucleus consists of a single proton. The deuterium nucleus has one proton and one neutron, while tritium has one proton and two neutrons.)
Bismuth-210 \(\left( {_{\;83}^{210}{\text{Bi}}} \right)\) is a radioactive isotope that decays as follows.
\[_{\;83}^{210}{\text{Bi}}\xrightarrow{{{\beta ^ – }}}{\text{X}}\xrightarrow{\alpha }{\text{Y}}\]
What are the mass number and proton number of Y?

Answer/Explanation
Markscheme
B
Bismuth-210 decays to polonium-210 through a beta decay.
alpha decay of polonium-210-

Question
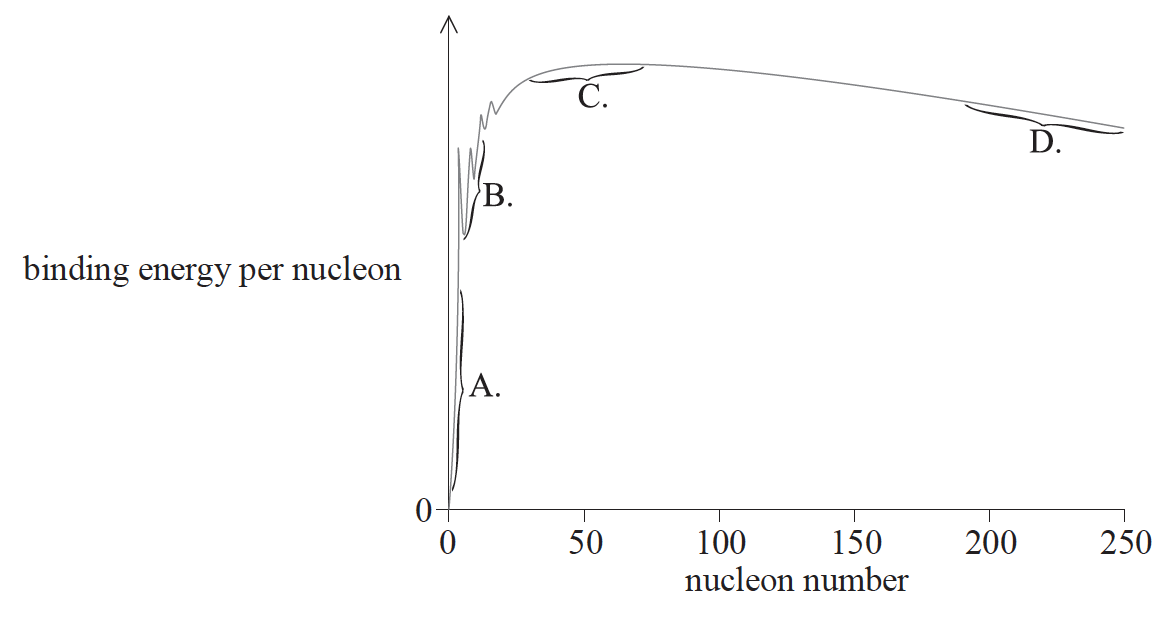
The graph shows the relationship between binding energy per nucleon and nucleon number. In which region are nuclei most stable?
Answer/Explanation
Markscheme
C
A sample contains an amount of radioactive material with a half-life of 3.5 days. After 2 weeks the fraction of the radioactive material remaining is
A. 94 %.
B. 25 %.
C. 6 %.
D. 0 %.
Answer/Explanation
Markscheme
C
\(t_{\frac{1}{2}}=\)3.5 days
in two weeks 14 dyas \(=4t_{\frac{1}{2}}\)
Material reamining after \(n\) half life \(=\frac{A_o}{2^n}\)
\(A_o =\) initial amount
After 4 half-life \(= \frac{A_o}{2^4}\rightarrow \frac{A_o}{16}\)
%age remaining \(=\frac{A_o/16}{A_o}\times 100\)
\(=6.25%\)