IB myp 4-5 physics – Practice Questions- All Topics
Topic :Atomic Physics-Atomic Structure,particles,charges and masses
Topic :Atomic Physics– Weightage : 21 %
All Questions for Topic :Atomic Structure,particles,charges and masses,Rdaioactivity and decay, forms of radiation, uses and damages
Understanding and using standard form
People regularly have to communicate large or small numbers and our language has words such as million or thousandth that help us to do this. The International System of Units, referred to as the SI system, also has prefixes which help communicate large or small units. For example, a kilometer is one thousand meters and a microgram is a millionth of a gram.
Some other prefixes used with SI units are shown below.

Scientists often need to express numbers which are beyond this scale. The mass of an electron is 0.91 thousandths of a yoctogram (the prefix yocto means 10–24 and is so small that it is rarely used) and so you would need about one million million million million million electrons to make a kilogram. Neither of these numbers is easy to communicate. Standard form makes it easier to represent large or small numbers. In standard form, we would write that the mass of an electron is 9.1 × 10–31 kg and so you would need just over 1 × 1030 electrons to make a kilogram.
Question:
Express these numbers in standard form:
a) The probability of shuffing a pack of cards and finding that they had ended up in sequential order is one in eighty million million million million million million million million million million million.
▶️Answer/Explanation
Ans: 8 × 1067
b) The number of insects on the Earth is estimated to be ten million million million.
▶️Answer/Explanation
Ans: 1 × 1019
c) The number of protons in the universe is thought to be about one hundred million million million million million million million million million million million million million.
▶️Answer/Explanation
Ans: 1 × 1080
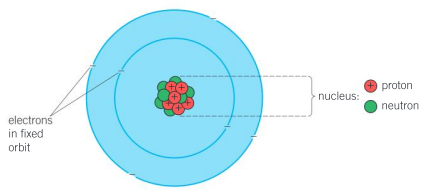
In Rutherford’s model of the atom, the nucleus consists of protons and neutrons, and the electrons are in fixed orbits around the nucleus. The overall atom has no charge, since there are the same number of electrons as protons
Question:
Why do you think that the electron was the easiest of these three particles to discover?
▶️Answer/Explanation
Ans: Electrons surround the nucleus. They are on the outside of the atom and so interact more easily with the outside world.