IB myp 4-5 physics – Practice Questions- All Topics
Topic :Atomic Physics-Radioactivity and Decay,forms of radiation, uses and damages
Topic :Atomic Physics– Weightage : 21 %
All Questions for Topic :Atomic Structure,particles,charges and masses,Rdaioactivity and decay, forms of radiation, uses and damages
Question (21 marks)
Developments in the understanding of atomic physics have led to many useful applications in industry, medicine and technology.
Through understanding patterns in the properties of alpha ( $\alpha)$, beta $(\beta)$ and gamma $(\gamma)$ radiation, scientists have developed ways in which the specific properties may be used.
One useful property is the difference in the penetration of alpha, beta and gamma radiation. The diagram compares the penetration of the three types of radiation.
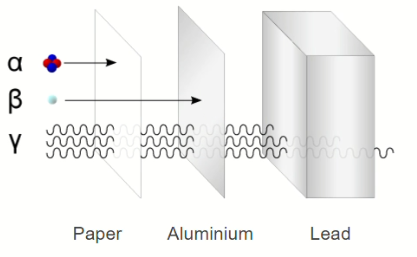
The amount of beta radiation absorbed depends on the thickness of the materials. This property of beta radiation is used to monitor the thickness of aluminium foil produced in an aluminium factory.
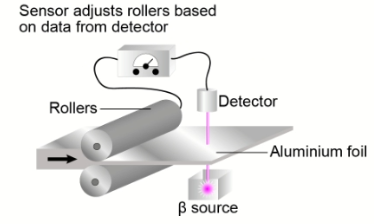
Question a (2 mark)
Your task is to design an investigation to determine how the thickness of aluminium foil affects the amount of beta radiation absorbed.
State the independent and dependent variables in your investigation.
Independent variable……
Dependent variable……..
▶️Answer/Explanation
Ans:
Independent variable: Thickness of the aluminium foil.
Dependent variable: Amount of beta radiation absorbed.
Question b (2 mark)
Outline the nature of beta radiation.
▶️Answer/Explanation
Ans:
Nature of beta radiation:
Beta radiation consists of high-energy electrons (beta particles) or positrons (positively charged electrons) that are emitted from the nucleus of an atom during radioactive decay. These particles have a negative charge and are much smaller and faster than alpha particles. Beta radiation can penetrate matter more deeply than alpha particles but less deeply than gamma radiation. It can be deflected by electric and magnetic fields, and its intensity can be reduced by absorptive materials, such as aluminum.
Question:
A sample of uranium-240 has an activity of 20,480Bq. After one week it has decayed until its activity is 5Bq. What is its half-life?
▶️Answer/Explanation
Ans: \(\frac{20480}{5}=4096;\)
212 = 4,096 so the activity of the sample has halved 12 times; \(half-life=\frac{168}{12}=12 hrs\)