IB myp 4-5 Chemistry – Practice Questions- All Topics
Topic :Bonding-Chemical Reactions
Topic :Bonding- Weightage : 21 %
All Questions for Topic :structure and bonding,properties,chemical formulas,chemical reactions and the conservation of mass; balancing,equations, the mole concept and chemical calculations;
reaction kinetics [rates, and factors affecting rates/collision theory],equilibria/reversible reactions,energy changes in reactions, endo- and exothermicity; combustion of fuels)
Question:
Calculate the amount (in moles) in each of the following masses:
a) 4.00 g of sodium hydroxide NaOH (Mr = 40.00 g mol−1)
b) 58.80 g of sulfuric acid H2SO4 (Mr = 98.09 g mol−1)
c) 14.6 g of hydrogen chloride HCl (calculate Mr)*
d) 58.8 g of nitric acid HNO3 (calculate Mr).*
*Use the values of Ar on the periodic table.
▶️Answer/Explanation
Ans: a) moles = mass (g) / molar mass (g mol–1)
= 4.00 / 40.00 = 0.100 mol
b) 58.80 / 98.09 = 0.5994 mol
c) 14.6 / 36.5 = 0.400 mol
d) 58.8 / 63.02 = 0.933 mol
Question:
Calculate the concentration, in mol dm−3, of the following:
a) 0.20 mol of nitric acid, HNO3, dissolved in water to make a 0.50 dm3 solution
b) 8.00 g of sodium hydroxide, NaOH, dissolved in water to make a 0.80 dm3 solution
c) 0.45 mol of sulfuric acid, H2SO4, dissolved in water to make a 0.75 dm3 solution
d) 6.80 g of ammonia, NH3, dissolved in water to make an 800 cm3 solution.
▶️Answer/Explanation
Ans: a) 0.20 / 0.50 = 0.40 mol dm–3
b) moles = 8.00 / 40.00 = 0.200 mol
0.200 / 0.80 = 0.25 mol dm–3
c) 0.45 / 0.75 = 0.60 mol dm–3
d) moles = 6.80 / 17.04 = 0.400 mol
0.400 / 0.800 = 0.499 mol dm–3
Question:
Two students are each asked to measure out three 9.5 g samples of a reactant. When their teacher checked their results, she found the following:
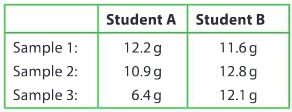
Comment on the level of accuracy and precision of both sets of data.
▶️Answer/Explanation
Ans: Student A results are not accurate as they are all different from the actual value of 9.5 g. The results are not precise as they are all different from each other. Student B results are not accurate as they are not close to the value of 9.5 g. However, they are precise as the three results are similar to each other.
