IB myp 4-5 Chemistry – Practice Questions- All Topics
Topic :Bonding-Reaction Kinetics
Topic :Bonding- Weightage : 21 %
All Questions for Topic :structure and bonding,properties,chemical formulas,chemical reactions and the conservation of mass; balancing,equations, the mole concept and chemical calculations;
reaction kinetics [rates, and factors affecting rates/collision theory],equilibria/reversible reactions,energy changes in reactions, endo- and exothermicity; combustion of fuels)
Question:
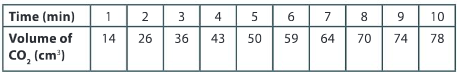
Plot the volume of carbon dioxide gas evolved V(CO2) against time t. You can do this by hand or by using graphing software. Remember that a smooth curve is required to represent the relationship between the data points.
▶️Answer/Explanation
Ans: 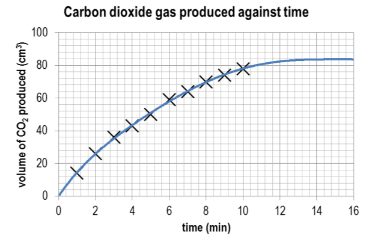
Question:
What conclusions can you draw about the progress of the reaction based on the shape of the curve?
▶️Answer/Explanation
Ans: The initial rate from 0 to 2 minutes is the fastest rate of reaction as this is the steepest part of the curve. As time progresses, the steepness of the curve decreases and therefore the rate of reaction is decreasing.
Question:
Draw a tangent to the curve at t=0 min and calculate the gradient of the tangent. What are the units for this rate?
▶️Answer/Explanation
Ans: Slope of tangent is approximately 19 cm3 min–1. Values given between 17 and 21 cm3 min–1 would be acceptable.