IB myp 4-5 Chemistry – Practice Questions- All Topics
Topic :Matter-Electron configuration and valency
Topic :Matter- Weightage : 21 %
All Questions for Topic : states and properties of matter,particle/kinetic theory,diffusion,atomic structure [including isotopes],electron configuration and valency
Question:
Determine the full electron configuration for the following atoms and ions:
a) sodium (Z = 11) b) aluminium (Z = 13)
c) chlorine (Z = 17) d) vanadium (Z = 23)
e) copper (Z = 29) f) bromine (Z = 35)
g) oxide ion, O2 ̄ (for h) magnesium ion, Mg2+
elemental oxygen, (for elemental
Z = 8) magnesium, Z = 12)
i) sulfide ion, S2 ̄ (for j) potassium ion, K+ (for
elemental sulfur, Z = 16) elemental potassium,
Z = 19)
▶️Answer/Explanation
Ans: a) 1s22s22p63s1
b) 1s22s22p63s23p1
c) 1s22s22p63s23p5
d) 1s22s22p63s23p64s23d3
e) 1s22s22p63s23p64s13d10
f) 1s22s22p63s23p64s23d104p5
g) 1s22s22p6
h) 1s22s22p6
i) 1s22s22p63s23p6
j) 1s22s22p63s23p6
Question:
All elements found in group 1 lose one valence electron when oxidized. What is the major difference between the electrons lost in each of these elements?
▶️Answer/Explanation
Ans: As you go down Group 1, the attraction between the valence electron and the positively charged nucleus decreases. The valence electron is less strongly held and the reactivity of the elements increases down Group 1.
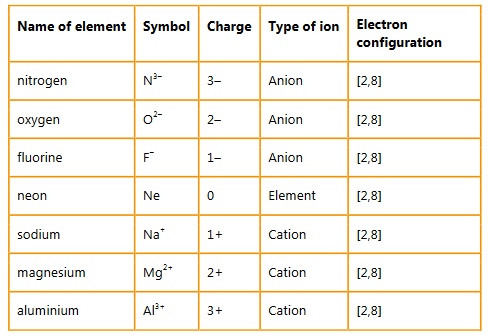
Question:
Give the names, symbols and charges of three cations and three anions that are isoelectronic with the noble gas neon [2,8].
▶️Answer/Explanation
Ans: