IB myp 4-5 Chemistry – Practice Questions- All Topics
Topic :The atmosphere -Alcohols
Topic :The atmosphere- Weightage : 21 %
All Questions for Topic : Characteristics of gases,Atmospheric composition,Testing and Treatment,Extraction,Emission and environmental Implications
The graph illustrates the changes in state for myristic acid, a saturated fatty acid, as it is heated. Saturated acids have been linked to high levels of cholesterol in humans and an increased chance of coronary heart disease.
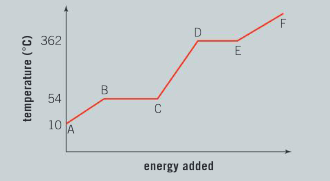
Question:
From your analysis of the graph:
a) Explain what is happening between B and C.
▶️Answer/Explanation
Ans: The region between b and c represents a solid/ liquid mixture; There is no increase in temperature in this region, as energy being added to the system is used to melt the solid myristic acid; when the change of state is complete and no more solid is present, the temperature will begin to rise again.
b) State the name used to describe this process.
▶️Answer/Explanation
Ans: Melting (or fusion).
c) Describe what is happening to the particles in this pure substance between C and D.
▶️Answer/Explanation
Ans: Heat energy is being added into the system from the surroundings resulting in an increase in temperature; the kinetic energy of the particles is increasing.
d) What is the boiling point of this liquid?
▶️Answer/Explanation
Ans: The boiling point of this liquid is 362°C.
e) The state of matter for this substance between E and F is a gas. Explain what will happen to the particles and the state of matter if energy is removed from the system.
▶️Answer/Explanation
Ans: If energy is removed from the system, the average kinetic energy of the particles decreases; particles move closer together and forces of attraction between the particles increases; there is a change of state from a gas to a liquid.
