IB myp 4-5 Chemistry – Practice Questions- All Topics
Topic :Types of chemical reaction-Acids and bases
Topic :Types of chemical reaction-Weightage : 21 %
All Questions for Topic :acids and bases,neutral solutions,acid/base reactions,$\mathrm{pH}$ and indicators,formation of salts,uses of salts,redox reactions,reactivity series,extraction of metals and corrosion,electrochemical cells
Question:
Consider the reactions of the following Group 3 oxides with water: sodium oxide, aluminium oxide, and sulfur dioxide. Describe whether each is acting as an acid or base. What does this suggest about the change in acid–base properties across a period?
▶️Answer/Explanation
Ans: Sodium oxide is acting as a basic oxide; aluminium oxide is amphoteric and can act as both a basic and acidic oxide; sulfur dioxide is acting as an acidic oxide; the general trend across period 3 of the periodic table is a change from basic to acidic oxides.
Question:
Construct a table and record your observations. Include the following:
a) the name and symbol of the reactants of each reaction
b) a description of the reaction including the color of any flame produced when the reactant was heated
c) the initial and final color
d) pH of the final reaction mixture.
▶️Answer/Explanation
Ans: 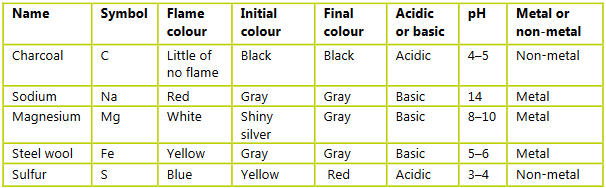
Question:
Using a universal indicator color strip, estimate the pH of each solution and record it in your data table.
▶️Answer/Explanation
Ans: 
