IB myp 4-5 Chemistry – Practice Questions- All Topics
Topic :Types of chemical reaction-$\mathrm{pH}$ and indicators
Topic :Types of chemical reaction-Weightage : 21 %
All Questions for Topic :acids and bases,neutral solutions,acid/base reactions,$\mathrm{pH}$ and indicators,formation of salts,uses of salts,redox reactions,reactivity series,extraction of metals and corrosion,electrochemical cells
Question:
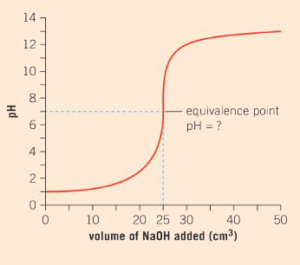
Comment on what the starting point of the pH curve on the y-axis tells us about the strength of hydrochloric acid?
▶️Answer/Explanation
Ans: The initial pH is 1.0 unit. This tells us that the acid is a strong acid.
Question:
Interpret the pH curve and explain why the pH of the solution starts to increase as sodium hydroxide is transferred from the burette to the conical flask.
▶️Answer/Explanation
Ans: The addition of sodium hydroxide will start the neutralization reaction. As the acid present in the flask is neutralized, the pH will start to rise as the solution becomes less acidic and more alkaline.
Question:
After 25 cm3 of sodium hydroxide has been transferred to the reaction mixture, there is a sudden increase in the pH level. What does this tell you about the amount of hydrochloric acid that remains unreacted in the conical flask? Justify your answer with scientific reasoning.
▶️Answer/Explanation
Ans: After 25 cm3 of sodium hydroxide has been transferred to the flask, no more hydrochloric acid will be present. This results in a sudden increase in the pH of the reaction mixture as the solution is now alkaline in nature.