Question
Two blocks \(\mathrm{X}\) and \(\mathrm{Y}\) at different temperatures are placed in thermal contact with each other until they reach thermal equilibrium. Block \(X\) and block \(Y\) are of the same material. The mass of block \(Y\) is half that of block \(X\). The change in temperature of block \(X\) has a magnitude \(\Delta T\) and the change in internal energy of block \(X\) has a magnitude \(\Delta U\). What is the change in magnitude of temperature of block \(Y\) and the change in magnitude of internal energy of block \(Y\) ?
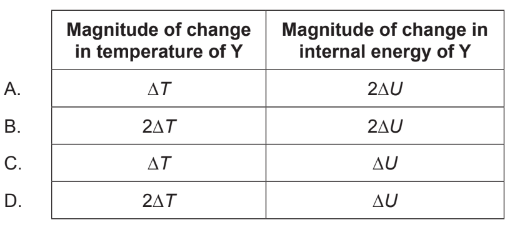
▶️Answer/Explanation
Ans:D
Question
A solid is heated at constant power in an insulated container. The graph shows the variation of temperature with time.

Why is the temperature constant for section QR?
A. The intermolecular potential energy of the molecules is constant.
B. The kinetic energy of the molecules is constant.
C. The internal energy of the solid is constant.
D. The rate at which the solid absorbs heat is equal to the rate at which it loses heat.
▶️Answer/Explanation
Ans:B
The correct answer is B. The kinetic energy of the molecules is constant.
In section QR of the graph, the temperature remains constant. When the temperature is constant, it means that the average kinetic energy of the molecules in the solid remains constant. This happens because the solid is being heated at a constant power, and the energy supplied to the solid is being used to increase the kinetic energy of the molecules rather than raising the temperature.
Option A is not correct because the intermolecular potential energy of the molecules can change even when the temperature is constant.
Option C is not correct because the internal energy of the solid can change with changes in kinetic energy and potential energy of the molecules.
Option D is not correct because it doesn’t specifically explain why the temperature remains constant; it’s a consequence of the fact that the kinetic energy is constant during this phase.
The pressure of a fixed mass of an ideal gas in a container is decreased at constant temperature. For the molecules of the gas there will be a decrease in
A. the mean square speed.
B. the number striking the container walls every second.
C. the force between them.
D. their diameter.
Answer/Explanation
Markscheme
B
The number of collisions between the gaseous molecules is directly related to the pressure of the gas. If the number of collisions (frequency of collisions) between the molecules is decreasing, then it will lead to a lower pressure of the gas in the container.
A sealed container contains a mixture of oxygen and nitrogen gas.
The ratio \(\frac{{{\text{mass of an oxygen molecule}}}}{{{\text{mass of a nitrogen molecule}}}}\) is \(\frac{8}{7}\).
The ratio \(\frac{{{\text{average kinetic energy of oxygen molecules}}}}{{{\text{average kinetic energy of nitrogen molecules}}}}\) is
A. 1.
B. \(\frac{7}{8}\).
C. \(\frac{8}{7}\).
D. dependent on the concentration of each gas.
Answer/Explanation
Markscheme
A
Average Kinetic energy of molecules is given by
\(KE =\frac{3RT}{2N_a}\) replacing \( (K=\frac{R}{N_a})\)
where R is gas constant
\(T\) is temperature (same for both oxygen and nitrogen as they are in the same sealed container)
\(N_a\) is Avogadro’s number
as average kinetic energy is independent of the mass of the molecule, the average kinetic energy of oxygen and nitrogen are same.
So the ratio will be 1
A sealed cylinder of length l and cross-sectional area A contains N molecules of an ideal gas at kelvin temperature T.

What is the force acting on the area of the cylinder marked A due to the gas?
A. \(\frac{{NRT}}{l}\)
B. \(\frac{{NRT}}{{lA}}\)
C. \(\frac{{N{k_B}T}}{{lA}}\)
D. \(\frac{{N{k_B}T}}{l}\)
Answer/Explanation
Markscheme
D
\(PV=nRT\)
and, \(K_b=\frac{R}{N_a}\)
Volume of cylinder \(= A\times l\)
\(P=\frac{N_aK_bT}{A\times l}\)
And, \(Pressure = Force\times Area\)
putting the value of P .
\(F=\frac{N_aK_bT}{ l}\)