Question
Which row represents the particles of a gas colliding most frequently?
Answer/Explanation
Ans: A
Question
Which test is used to show that a sample of water is pure?
A Evaporate the water to see if any solids remain.
B Heat the water to check its boiling point.
C Test with anhydrous cobalt(II) chloride.
D Use universal indicator paper to check its pH.
Answer/Explanation
Ans: B
Question
Which piece of apparatus is used to measure 1.5 \(cm^3\) of a solution accurately?
A 25 \(cm^3\) measuring cylinder
B 25 \(cm^3\) pipette
C 50 \(cm^3\) measuring cylinder
D 50 \(cm^3\) burette
Answer/Explanation
Ans: D
Question
A representation of an atom is shown.
What is the nucleon number of this atom?
A 6 B 7 C 12 D 13
Answer/Explanation
Ans: D
Question
Lithium reacts with fluorine to form the compound lithium fluoride.
Which statement about this reaction is correct?
A Each fluorine atom gains one electron.
B Each fluorine atom gains two or more electrons.
C Each fluorine atom loses one electron.
D Each fluorine atom loses two or more electrons.
Answer/Explanation
Ans: A
Question
Ammonia, \(NH_3\), is a covalent molecule.
Which diagram shows the outer shell electron arrangement in a molecule of ammonia?
Answer/Explanation
Ans: C
Question
Which row describes the structure and a use of diamond?
Answer/Explanation
Ans: C
Question
Methane, \(CH_4\), burns in air to form carbon dioxide and water.
What is the balanced equation for this reaction?
A \(CH_4(g) + O_2(g) → CO_2(g) + 2H_2O(g)\)
B \(CH_4(g) + 2O_2(g) → CO_2(g) + 2H_2O(g)\)
C \(CH_4(g) + 2O_2(g) → CO_2(g) + H_2O(g)\)
D \(CH_4(g) + 3O_2(g) → CO_2(g) + 2H_2O(g)\)
Answer/Explanation
Ans: B
Question
Which statement about electrolysis is correct?
A Chemical energy is converted to electrical energy.
B Electrons flow through the electrolyte.
C Ionic compounds are broken down.
D Metals are formed at the positive electrode.
Answer/Explanation
Ans: C
Question
Which energy level diagram shows the reaction that will give out the most energy?
Answer/Explanation
Ans: A
Question
Which change is a physical change?
A Copper(II) carbonate changes colour from green to black when it is heated, and stays black when it cools.
B Ethanol reacts with oxygen to form carbon dioxide and water.
C Hydrogen peroxide decomposes into water and oxygen when it is boiled.
D Ice forms liquid water when it is heated.
Answer/Explanation
Ans: D
Question
Marble chips (calcium carbonate) react with hydrochloric acid in an exothermic reaction.
calcium carbonate + hydrochloric acid → calcium chloride + water + carbon dioxide
When excess marble chips are added to dilute hydrochloric acid the rate of the reaction starts off
fast, then gets slower until the reaction stops.
Why does the reaction rate get slower?
A The concentration of the hydrochloric acid is decreasing.
B The concentration of calcium chloride is increasing.
C The calcium carbonate is completely used up.
D The temperature of the mixture decreases.
Answer/Explanation
Ans: A
Question
The diagram shows the change from an anhydrous salt to its hydrated form.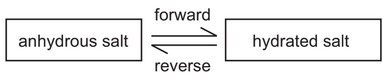
Which statement is correct?
A The forward reaction requires heat and water.
B The forward reaction requires water only.
C The reverse reaction requires heat and water.
D The reverse reaction requires water only.
Answer/Explanation
Ans: B
Question
A violent reaction occurs when a mixture of chromium(III) oxide and aluminium is ignited with a
magnesium fuse as shown.
Which substance is oxidised in the reaction?
A aluminium
B aluminium oxide
C chromium
D chromium(III) oxide
Answer/Explanation
Ans: A
Question
A farmer’s soil is acidic.
Which substance should the farmer add to neutralise the soil?
A ammonium sulfate
B calcium oxide
C hydrochloric acid
D NPK fertiliser
Answer/Explanation
Ans: B
Question
Three elements, X, Y and Z, are burned in oxygen.
The oxides formed are dissolved in water and the pH of the solutions measured.
The results are shown.
Which statements are correct?
1 Element X could be sulfur.
2 Element Y could be sodium.
3 Element Z is a non-metal.
4 No metal elements were used.
A 1 only B 1 and 2 C 2 and 3 D 3 and 4
Answer/Explanation
Ans: B
Question
The following substances can be reacted together to prepare salts.
1 copper(II) oxide and excess hydrochloric acid
2 hydrochloric acid and excess sodium hydroxide
3 hydrochloric acid and excess zinc carbonate
In which reactions can the excess reactant be separated from the solution by filtration?
A 1 and 2 B 1 and 3 C 2 and 3 D 3 only
Answer/Explanation
Ans: D
Question
Salt S is dissolved in water and three tests are carried out on the solution.
What is the identity of S?
A copper(II) chloride
B copper(II) sulfate
C iron(II) chloride
D iron(II) sulfate
Answer/Explanation
Ans: D
Question
Which statement about the Periodic Table is correct?
A Most metallic elements are on the left.
B Elements in the same period have the same number of outer electrons.
C Elements on the left are usually gases.
D The relative atomic mass of the elements increases from right to left.
Answer/Explanation
Ans: A
Question
The diagram shows elements W, X, Y and Z in a section of the Periodic Table.
Which statement about the reactivity of the elements is correct?
A X is more reactive than Y, and W is more reactive than Z.
B X is more reactive than Y, and Z is more reactive than W.
C Y is more reactive than X, and W is more reactive than Z.
D Y is more reactive than X, and Z is more reactive than W.
Answer/Explanation
Ans: C
Question
Some properties of substances are listed.
1 They conduct electricity.
2 They have low densities.
3 They have high melting points.
4 They are malleable.
Which properties are shown by transition metals?
A 1 and 3 only B 1 and 4 only C 1, 2 and 3 D 1, 3 and 4
Answer/Explanation
Ans: D
Question
Which statement about the noble gas argon is correct?
A It burns with a hot flame.
B It is used in airships because of its low density.
C It exists as diatomic molecules.
D It has eight electrons in its outermost shell.
Answer/Explanation
Ans: D
Question
Sodium is a Group I metal.
Which property, that is typical of most metals, is not shown by sodium?
A conductor of heat
B high melting point
C malleable
D shiny
Answer/Explanation
Ans: B
Question
Manganese, nickel and silver are all metals.
Samples of powdered manganese, nickel and silver were placed in separate test-tubes
containing dilute hydrochloric acid.
The results are shown.
What is the order of reactivity of the metals, most reactive to least reactive?
A manganese → nickel → silver
B manganese → silver → nickel
C silver → manganese → nickel
D silver → nickel → manganese
Answer/Explanation
Ans: A
Question
Which statement about aluminium is correct?
A Aluminium is easy to extract from its ore because it is near the bottom of the reactivity series.
B Aluminium is formed when aluminium oxide is heated with carbon.
C Bauxite is an important ore of aluminium.
D Hematite is an important ore of aluminium.
Answer/Explanation
Ans: C
Question
Some properties of aluminium are listed.
1 It conducts heat.
2 It has a low density.
3 It is strong.
4 It is resistant to corrosion.
Which of these properties make aluminium suitable for making food containers for chilled food
products?
A 1, 2 and 4 B 1, 3 and 4 C 1 only D 4 only
Answer/Explanation
Ans: D
Question
Water is treated at a waterworks to make it fit to drink.
What is present in the water when it leaves the waterworks?
A bacteria only
B bacteria and insoluble substances
C chlorine compounds only
D chlorine compounds and soluble substances
Answer/Explanation
Ans: D
Question
Sulfur dioxide, carbon monoxide and oxides of nitrogen are common gaseous pollutants found in the air.
Which pollutants contribute to acid rain?
A carbon monoxide and sulfur dioxide
B oxides of nitrogen and sulfur dioxide
C oxides of nitrogen only
D sulfur dioxide only
Answer/Explanation
Ans: B
Question
Which methods prevent iron from rusting?
Answer/Explanation
Ans: C
Question
Fertilisers are mixtures of different compounds used to increase the growth of crops.
Which pair of substances contain the three essential elements for plant growth?
A ammonium nitrate and calcium phosphate
B ammonium nitrate and potassium chloride
C ammonium phosphate and potassium chloride
D potassium nitrate and calcium carbonate
Answer/Explanation
Ans: C
Question
Which process does not add a greenhouse gas to the atmosphere?
A burning methane
B decomposition of vegetation
C polymerisation
D respiration
Answer/Explanation
Ans: C
Question
Why is sulfur dioxide used as a food preservative?
A It is a gas at room temperature.
B It is used to make sulfuric acid.
C It kills bacteria.
D It reacts with alkalis.
Answer/Explanation
Ans: C
Question
Which statements about lime (calcium oxide) and limestone (calcium carbonate) are correct?
1 Limestone is used in the manufacture of iron.
2 Lime is made by heating limestone.
3 Powdered limestone is heated with clay in the production of cement.
4 Limestone causes soil to be acidic.
A 1 and 2 only B 2 and 3 only C 1, 2 and 3 D 1, 3 and 4
Answer/Explanation
Ans: C
Question
The formulae of two organic compounds, P and Q, are shown.
Answer/Explanation
Ans: B
Question
Petroleum is an important raw material that is separated into useful products.
Which terms describe petroleum and the method used to separate it?
Answer/Explanation
Ans: D
Question
Which type of compound is a member of a homologous series?
A carbonate
B carboxylic acid
C halogen
D hydroxide
Answer/Explanation
Ans: B
Question
Which statements about propene are correct?
1 Propene contains only single bonds.
2 Propene decolourises bromine water.
3 Propene is obtained by cracking.
4 Propene is a hydrocarbon.
A 1 and 4 B 2, 3 and 4 C 2 and 4 only D 4 only
Answer/Explanation
Ans: B
Question
Which row describes the production of ethanol and its properties?
Answer/Explanation
Ans: A
Question
Which statements about ethanoic acid are correct?
1 It contains a carbon–oxygen double bond.
2 It contains two carbon atoms.
3 It decolourises bromine water.
4 It contains an –OH group.
A 1 and 2 only B 1 and 3 C 1, 2 and 4 D 2, 3 and 4
Answer/Explanation
Ans: C
Question
Which polymers are natural polymers?
1 carbohydrates
2 poly(ethene)
3 protein
A 1, 2 and 3 B 1 and 3 only C 1 only D 3 only
Answer/Explanation
Ans: B
