Question
Which change of state is an exothermic process?
A. condensation
B. evaporation
C. melting
D. sublimation
Answer/Explanation
Ans:
A
Question
In which state does 1 \(dm^{3}\) of methane contain the most particles?
A. gas at \(100^{\circ}\)C
B. gas at room temperature
C. liquid
D. solid
Answer/Explanation
Ans:
D
Question
Which dye on the chromatogram is a pure substance?

Answer/Explanation
Ans:
A
Question
Which piece of apparatus is used to measure exactly 5.00 \(cm^{3}\) of a liquid?
A. 5 \(cm^{3}\) beaker
B. 10 \(cm^{3}\) measuring cylinder
C. 25 \(cm^{3}\) pipette
D. 50 \(cm^{3}\) burette
Answer/Explanation
Ans:
D
Question
Fermentation of sugar produces a mixture of ethanol solution and solid yeast. How is the solid yeast removed from the mixture?
A. crystallisation
B. distillation
C. filtration
D. fractional distillation
Answer/Explanation
Ans:
C
Question
Matter exists as elements, compounds and mixtures. Which row identifies an element, a compound and a mixture?

Answer/Explanation
Ans:
C
Question
Which pair of statements about diamond and graphite is correct?
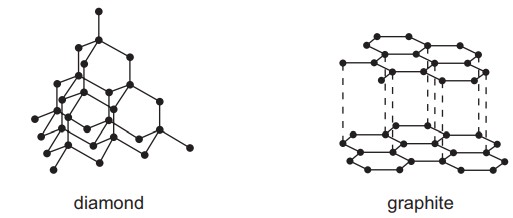
A. Diamond and graphite are both pure carbon. They are both macromolecules.
B. Diamond and graphite can both be used as electrodes. Graphite is also used as a lubricant.
C. Diamond has covalent bonds. Graphite has ionic bonds.
D. Diamond is hard with a high melting point. Graphite is soft with a low melting point.
Answer/Explanation
Ans:
A
Question
An isotope of chromium is represented by \(_{24}^{52}\textrm{Cr}\). Which statement about an atom of this isotope of chromium is correct?
A. It contains 24 electrons.
B. It contains 24 neutrons.
C. It contains 28 protons.
D. It contains 52 neutrons.
Answer/Explanation
Ans:
A
Question
Sodium is in Group I of the Periodic Table and chlorine is in Group VII. Which row describes what happens when sodium bonds ionically with chlorine?
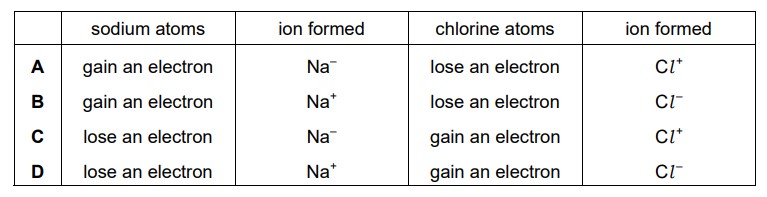
Answer/Explanation
Ans:
D
Question
Caesium fluoride is an ionic compound. Which statements about caesium fluoride are correct?
1. It conducts electricity when solid.
2. It has a high melting point.
3. It is soluble in water.
4. It is highly volatile.
A. 1 and 2 B. 1 and 4 C. 2 and 3 D. 3 and 4
Answer/Explanation
Ans:
C
Question
The structure of a molecule of a compound is shown.

What is the formula of this compound?
A. \(C_{3}H_{7}O\) B. \(C_{3}H_{8}O\) C. \(C_{8}H_{3}O\) D. \(C_{8}HO_{3}\)
Answer/Explanation
Ans:
B
Question
Calcium carbonate, CaCO3, reacts with dilute hydrochloric acid to produce carbon dioxide. The equation for the reaction is shown. The relative formula mass of calcium carbonate is 100.
\(CaCO_{3}+2HC\imath \rightarrow CaC\imath _{2}+H_{2}O+CO_{2}\)
10 g of calcium carbonate is reacted with an excess of dilute hydrochloric acid. Which mass of carbon dioxide is produced?
A. 2.2 g B. 2.8 g C. 4.4 g D. 44 g
Answer/Explanation
Ans:
C
Question
Molten sodium chloride and concentrated aqueous sodium chloride are electrolysed using platinum electrodes. What are the products at the negative electrode (cathode) in each electrolysis?
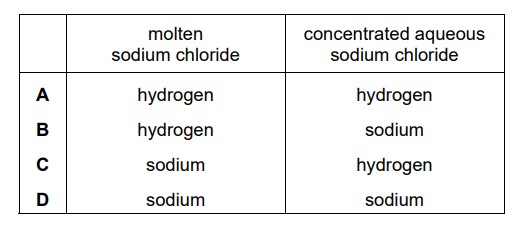
Answer/Explanation
Ans:
C
Question
An object is electroplated with silver using an aqueous silver salt as the electrolyte. Which row is correct?
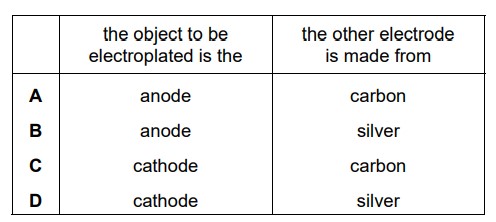
Answer/Explanation
Ans:
D
Question
Which row describes the changes that occur in an endothermic reaction?

Answer/Explanation
Ans:
C
Question
Which statement about fuels is correct?
A. Heat energy is only produced by burning fuels.
B. Hydrogen is used as a fuel although it is difficult to store.
C. Methane is a good fuel because it produces only water when burned.
D. Uranium is burned in air to produce energy.
Answer/Explanation
Ans:
B
Question
A sequence of changes involving sulfur is shown.
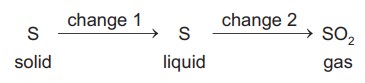
Which row describes the changes?
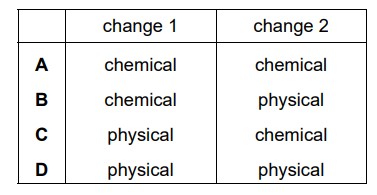
Answer/Explanation
Ans:
C
Question
Magnesium is added to dilute hydrochloric acid. 25 \(cm^{3}\) of gas is given off in the first 30 s of the reaction. The experiment is repeated at a lower temperature. All other reaction conditions are the same. Which volume of gas is produced in the first 30 s of this reaction?
A. 15 \(cm^{3}\) B. 25 \(cm^{3}\) C. 30 \(cm^{3}\) D. 50 \(cm^{3}\)
Answer/Explanation
Ans:
A
Question
The equation for the reaction between magnesium and copper(II) oxide is shown.
\(Mg+CuO\rightarrow MgO+Cu\)
Which substance is oxidised?
A. Cu B. CuO C. Mg D. MgO
Answer/Explanation
Ans:
C
Question
Methyl orange is added to dilute hydrochloric acid and to aqueous sodium hydroxide. What is the colour of the methyl orange in each solution?
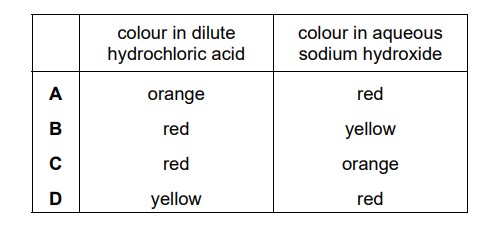
Answer/Explanation
Ans:
B
Question
Compound X is dissolved in water and two separate samples of the solution are tested. The results of the tests are shown.
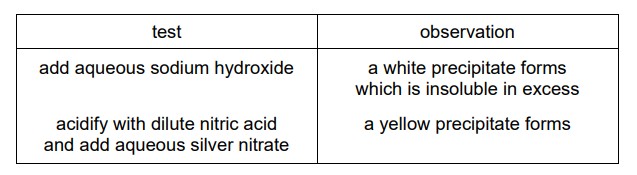
What is compound X?
A. calcium chloride
B. calcium iodide
C. zinc chloride
D. zinc iodide
Answer/Explanation
Ans:
B
Question
Which statement about the Periodic Table is correct?
A. Elements with the highest atomic number in each period are metallic.
B. Elements with the lowest group numbers are non-metals.
C. Elements with similar chemical properties are placed in groups.
D. Elements with similar physical properties are placed in periods.
Answer/Explanation
Ans:
C
Question
Part of the Periodic Table is shown. Which element is a soft solid that reacts violently with cold water?
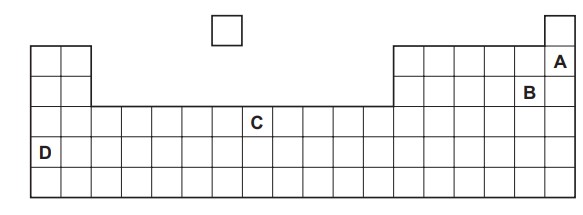
Answer/Explanation
Ans:
D
Question
Three properties of element X are listed.
● It contains atoms with a full outer shell of electrons.
● It is monoatomic.
● It is unreactive.
In which part of the Periodic Table is the element placed?
A. Group I
B. Group VII
C. Group VIII
D. transition elements
Answer/Explanation
Ans:
C
Question
Some properties of the elements in Group VII of the Periodic Table are shown.
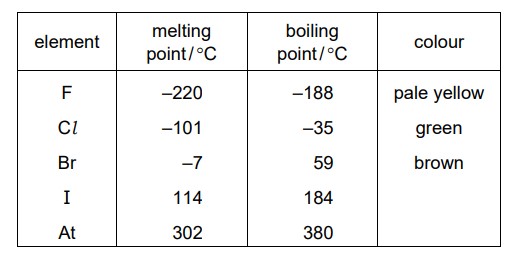
Which statement is correct?
A. Bromine is a brown solid at room temperature.
B. Fluorine is a pale yellow gas at room temperature.
C. Iodine is a brown liquid at room temperature.
D. Astatine is a black liquid at room temperature.
Answer/Explanation
Ans:
B
Question
Which process is used to obtain the metal calcium from its ore?
A. electrolysis
B. oxidation with carbon
C. reduction with carbon
D. thermal decomposition
Answer/Explanation
Ans:
A
Question
Which row links the property of a metal to its use?

Answer/Explanation
Ans:
D
Question
The table gives some properties of an element.

Which other property does this element have?
A. acts as a catalyst
B. brittle
C. forms an acidic oxide
D. highly reactive with water
Answer/Explanation
Ans:
A
Question
A metal reacts vigorously with cold water. Which statement about the metal is correct?
A. It is above hydrogen in the reactivity series.
B. It is below magnesium in the reactivity series.
C. Its oxide can be reduced with carbon.
D. It does not react with dilute acids.
Answer/Explanation
Ans:
A
Question
Which row describes the colour changes when water is added to anhydrous cobalt(II) chloride and anhydrous copper(II) sulfate?
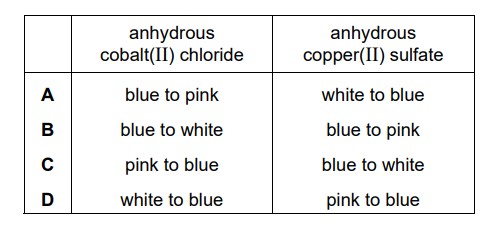
Answer/Explanation
Ans:
A
Question
The gases produced by a burning fuel are passed through solution Z using the apparatus shown. The fuel contains compounds of sulfur.

Which row identifies solution Z and the result obtained when the fuel contains compounds of sulfur?

Answer/Explanation
Ans:
A
Question
Which information about carbon dioxide and methane is correct?
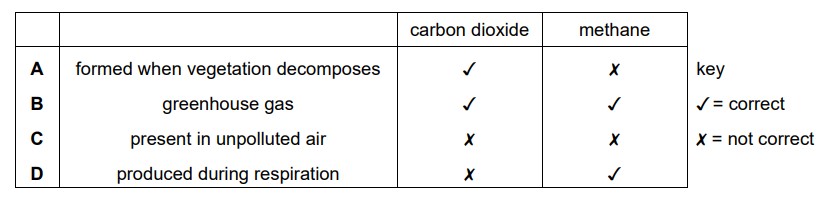
Answer/Explanation
Ans:
B
Question
Which row identifies uses of sulfur?
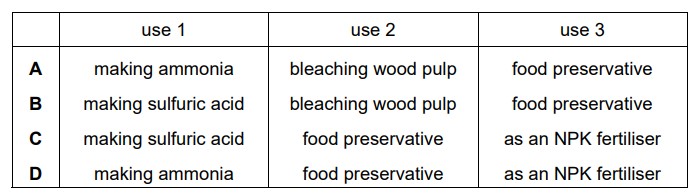
Answer/Explanation
Ans:
B
Question
Which statements about lime are correct?
1 Lime is made by heating calcium carbonate (limestone).
2 Lime is used to desulfurise flue gases.
3 Lime is used to treat alkaline soil.
4 The chemical name for lime is calcium oxide.
A. 1 and 3 B. 1, 2 and 4 C. 1 and 4 only D. 2, 3 and 4
Answer/Explanation
Ans:
B
Question
Which structure is correctly named?

Answer/Explanation
Ans:
C
Question
The fractional distillation of petroleum produces a series of fractions with different uses. Which row identifies a use for a fraction?

Answer/Explanation
Ans:
D
Question
Ethene and propene are both members of the same homologous series. Which statements explain why ethene and propene have similar chemical properties?
1 They are both hydrocarbons.
2 They are both made by cracking.
3 They have the same functional group.
A. 1 and 2 B. 1 and 3 C. 2 only D. 3 only
Answer/Explanation
Ans:
D
Question
Which statement about ethane is correct?
A. It decolourises bromine water.
B. It burns in excess oxygen to form water and carbon dioxide.
C. Its molecular formula is C2H4.
D. Its atoms are joined together by ionic bonding.
Answer/Explanation
Ans:
B
Question
Which statements about ethanol are correct?
1 Ethanol is used as a solvent.
2 Ethanol can be made directly from ethane.
3 Ethanol is a covalent compound.
A. 1 only B. 1 and 2 C. 1 and 3 D. 2 and 3
Answer/Explanation
Ans:
C
Question
Polymers are long-chain molecules made from small molecules linked together. Four polymers or types of polymer are listed.
1 carbohydrates
2 nylon
3 proteins
4 Terylene
Which polymers or types of polymer are synthetic?
A. 1 and 3 B. 1 and 4 C. 2 and 3 D. 2 and 4
Answer/Explanation
Ans:
D
