Question
Which gas has the fastest rate of diffusion?
A. \(H_{2}\) B. \(CH_{4}\) C. \(CO_{2}\) D. \(SO_{2}\)
Answer/Explanation
Ans:
A
Question
In which state does 1 \(dm^{3}\) of methane contain the most particles?
A. gas at \(100^{\circ}C\)
B. gas at room temperature
C. liquid
D. solid
Answer/Explanation
Ans;
D
Question
Which statement explains why isotopes of the same element have the same chemical properties?
A. They have the same electronic structure.
B. They have the same relative mass.
C. They have the same nucleon number.
D. They have the same proton number.
Answer/Explanation
Ans:
A
Question
The electronic structures of atoms P and Q are shown.

P and Q form an ionic compound. What is the formula of the compound?
A. PQ B. \(P_{2}Q\) C. \(P_{2}Q_{3}\) D. \(PQ_{2}\)
Answer/Explanation
Ans:
D
Question
Fermentation of sugar produces a mixture of ethanol solution and solid yeast. How is the solid yeast removed from the mixture?
A. crystallisation
B. distillation
C. filtration
D. fractional distillation
Answer/Explanation
Ans:
C
Question
Which row explains why copper is a good conductor of electricity at room temperature?

Answer/Explanation
Ans:
B
Question
Which pair of statements about diamond and graphite is correct?

A. Diamond and graphite are both pure carbon. They are both macromolecules.
B. Diamond and graphite can both be used as electrodes. Graphite is also used as a lubricant.
C. Diamond has covalent bonds. Graphite has ionic bonds.
D. Diamond is hard with a high melting point. Graphite is soft with a low melting point.
Answer/Explanation
Ans:
A
Question
Sodium nitride contains the nitride ion, \(N^{3-}\). Sodium nitride is unstable and decomposes into its elements. What is the equation for the decomposition of sodium nitride?
A. \(2NaN_{3}\rightarrow 2Na+3N_{2}\)
B. \(2Na_{3}N\rightarrow 6Na+N_{2}\)
C. \(2NaN_{3}\rightarrow Na_{2}+3N_{2}\)
D. \(2Na_{3}N\rightarrow 6Na+2N\)
Answer/Explanation
Ans:
B
Question
Compound X contains carbon, hydrogen and oxygen only. By mass, it contains 26.7% carbon and 2.2% hydrogen. What is the empirical formula of X?
A. CHO B. \(C_{2}HO\) C. \(CH_{2}O\) D. \(CHO_{2}\)
Answer/Explanation
Ans:
D
Question
Caesium fluoride is an ionic compound. Which statements about caesium fluoride are correct?
1 It conducts electricity when solid.
2 It has a high melting point.
3 It is soluble in water.
4 It is highly volatile.
A. 1 and 2 B. 1 and 4 C. 2 and 3 D. 3 and 4
Answer/Explanation
Ans:
C
Question
Which diagram shows the direction of movement of ions and electrons during the electrolysis of molten sodium chloride?

Answer/Explanation
Ans:
A
Question
Calcium carbonate, \(CaCO_{3}\), reacts with dilute hydrochloric acid to produce carbon dioxide. The equation for the reaction is shown. The relative formula mass of calcium carbonate is 100.
\(CaCO_{3}+2HC\imath \rightarrow CaC\imath _{2}+H_{2}O+CO_{2}\)
10 g of calcium carbonate is reacted with an excess of dilute hydrochloric acid. Which mass of carbon dioxide is produced?
A. 2.2 g B. 2.8 g C. 4.4 g D. 44 g
Answer/Explanation
Ans:
C
Question
Molten sodium chloride and concentrated aqueous sodium chloride are electrolysed using platinum electrodes. What are the products at the negative electrode (cathode) in each electrolysis?

Answer/Explanation
Ans:
C
Question
An object is electroplated with silver using an aqueous silver salt as the electrolyte. Which row is correct?

Answer/Explanation
Ans:
D
Question
Which row describes the changes that occur in an endothermic reaction?

Answer/Explanation
Ans:
C
Question
Which statement about fuels is correct?
A. Heat energy is only produced by burning fuels.
B. Hydrogen is used as a fuel although it is difficult to store.
C. Methane is a good fuel because it produces only water when burned.
D. Uranium is burned in air to produce energy.
Answer/Explanation
Ans:
B
Question
Which statement about endothermic and exothermic reactions is correct?
A. In an endothermic reaction, less energy is absorbed in bond breaking than is released in bond forming.
B. In an endothermic reaction, the activation energy is always higher than in an exothermic reaction.
C. In an exothermic reaction, more energy is absorbed in bond breaking than is released in bond forming.
D. In an exothermic reaction, the reactants are higher on an energy level diagram than the products.
Answer/Explanation
Ans:
D
Question
The reaction used to manufacture ammonia from nitrogen and hydrogen is reversible. An equilibrium is established between ammonia, nitrogen and hydrogen. Which statement describes the equilibrium?
A. Both the forward reaction and the backward reaction have the same rate.
B. The rate of the backward reaction is greater than the rate of the forward reaction.
C. The rate of the forward reaction is greater than the rate of the backward reaction.
D. The forward and backward reactions have both stopped.
Answer/Explanation
Ans:
A
Question
How does increasing the concentration affect the reacting particles in a chemical reaction?
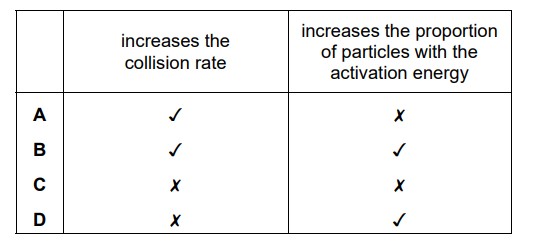
Answer/Explanation
Ans:
A
Question
Methyl orange is added to dilute hydrochloric acid and to aqueous sodium hydroxide. What is the colour of the methyl orange in each solution?

Answer/Explanation
Ans:
B
Question
Zinc oxide is an amphoteric oxide. Which types of substances will react with zinc oxide?
A. acids and bases
B. acids only
C. bases only
D. neither acids nor bases
Answer/Explanation
Ans:
A
Question
Information about some silver compounds is shown.

Which equation shows a reaction which cannot be used to make a silver salt?
A. \(AgNO_{3}(aq)+HC\imath (aq)\rightarrow AgC\imath (s)+HNO_{3}(aq)\)
B. \(Ag_{2}O(s)+2HNO_{3}(aq)\rightarrow 2AgNO_{3}(aq)+H_{2}O(l)\)
C. \(Ag_{2}CO_{3}(s)+2HNO_{3}(aq)\rightarrow 2AgNO_{3}(aq)+H_{2}O(l)+CO_{2}(g)\)
D. \(2Ag(s)+2HC\imath (aq)\rightarrow 2AgC\imath (s)+H_{2}(g)\)
Answer/Explanation
Ans:
D
Question
Aqueous ethanoic acid is a weak acid. Aqueous sodium hydroxide is a strong base. Aqueous ethanoic acid is neutralised by aqueous sodium hydroxide. Which statements are correct?
1 Aqueous ethanoic acid accepts protons from hydroxide ions.
2 The aqueous ethanoic acid used is fully dissociated into ions.
3 The aqueous sodium hydroxide used is fully dissociated into ions.
4 The reaction produces a salt and water.
A. 1 and 2 B. 1 and 3 C. 2 and 4 D. 3 and 4
Answer/Explanation
Ans:
D
Question
Three properties of element X are listed.
● It contains atoms with a full outer shell of electrons.
● It is monoatomic.
● It is unreactive.
In which part of the Periodic Table is the element placed?
A. Group I
B. Group VII
C. Group VIII
D. transition elements
Answer/Explanation
Ans:
C
Question
Which word equation represents a reaction that occurs?
A. sodium oxide + carbon \(\rightarrow\) sodium + carbon dioxide
B. sodium oxide + iron \(\rightarrow\) sodium + iron(II) oxide
C. iron(II) oxide + copper \(\rightarrow\) iron + copper(II) oxide
D. iron(III) oxide + carbon \(\rightarrow\) iron + carbon dioxide
Answer/Explanation
Ans:
D
Question
Which statement about the extraction of aluminium is correct?
A. Aluminium is formed at the cathode during the electrolysis of aluminium oxide.
B. Hematite is mainly aluminium oxide.
C. Molten cryolite is used to raise the melting point of the aluminium oxide.
D. Oxygen gains electrons at the anode during the electrolysis of aluminium oxide.
Answer/Explanation
Ans:
A
Question
Metal M is mixed with copper to produce brass. What is M?
A. chromium
B. nickel
C. vanadium
D. zinc
Answer/Explanation
Ans:
D
Question
The table gives some properties of an element.

Which other property does this element have?
A. acts as a catalyst
B. brittle
C. forms an acidic oxide
D. highly reactive with water
Answer/Explanation
Ans:
A
Question
Ammonia is produced using the Haber process. Which row shows the source of the raw materials and the reaction conditions?

Answer/Explanation
Ans:
C
Question
How many species are acting as bases in this reversible reaction?
\(HNO_{3}+H_{2}O\rightleftharpoons H_{3}O^{+}+NO_{3}^{-}\)
A. 3 B. 2 C. 1 D. 0
Answer/Explanation
Ans:
B
Question
The equation for a reaction occurring in the Contact process is shown.
\(2SO_{2}+O_{2}\rightarrow 2SO_{3}\)
What is the catalyst used in this reaction?
A. iron
B. phosphoric(V) acid
C. sulfuric acid
D. vanadium(V) oxide
Answer/Explanation
Ans:
D
Question
Which information about carbon dioxide and methane is correct?

Answer/Explanation
Ans:
B
Question
The structure of an ester is shown.

What are the names of the carboxylic acid and the alcohol that react together to form this ester?

Answer/Explanation
Ans:
B
Question
Which statements about lime are correct?
1 Lime is made by heating calcium carbonate (limestone).
2 Lime is used to desulfurise flue gases.
3 Lime is used to treat alkaline soil.
4 The chemical name for lime is calcium oxide.
A. 1 and 3 B. 1, 2 and 4 C. 1 and 4 only D. 2, 3 and 4
Answer/Explanation
Ans:
B
Question
Which structure is correctly named?

Answer/Explanation
Ans:
C
Question
The structure of part of a polymer is shown.

Which monomers can be used to make this polymer?

A. 1 and 2 B. 1 and 4 C. 2 and 3 D. 3 and 4
Answer/Explanation
Ans:
A
Question
Carboxylic acids are made by the oxidation of alcohols. Which carboxylic acid is produced from \(CH_{3}CH_{2}OH\)?
A. butanoic acid
B. ethanoic acid
C. methanoic acid
D. propanoic acid
Answer/Explanation
Ans:
B
Question
Propene, \(C_{3}H_{6}\), reacts with bromine, \(Br_{2}\), in an addition reaction. Which structure represents the product of this reaction?

Answer/Explanation
Ans:
C
Question
Which statements about ethanol are correct?
1 Ethanol is used as a solvent.
2 Ethanol can be made directly from ethane.
3 Ethanol is a covalent compound.
A. 1 only B. 1 and 2 C. 1 and 3 D. 2 and 3
Answer/Explanation
Ans:
C
Question
Which diagram represents the structure of nylon?

Answer/Explanation
Ans:
C
