The diagrams show containers of gas at the same temperature. All containers have the same size.
Which container contains gas at the highest pressure?
A) 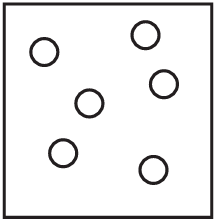
B) 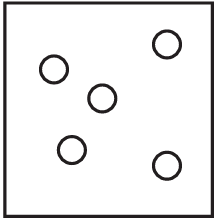
C) 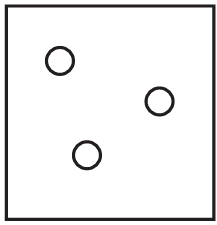
D) 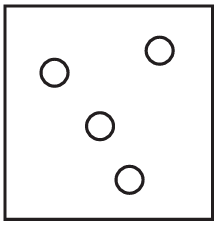
▶️ Answer/Explanation
Ans: A
Pressure is determined by the number of gas particles colliding with the container walls when temperature and volume are constant. Container A has the most particles (5), meaning more collisions per unit time, resulting in higher pressure. The other containers have fewer particles (B has 4, C has 3, D has 2), so they would have lower pressures.
A cooling curve for a substance is shown.
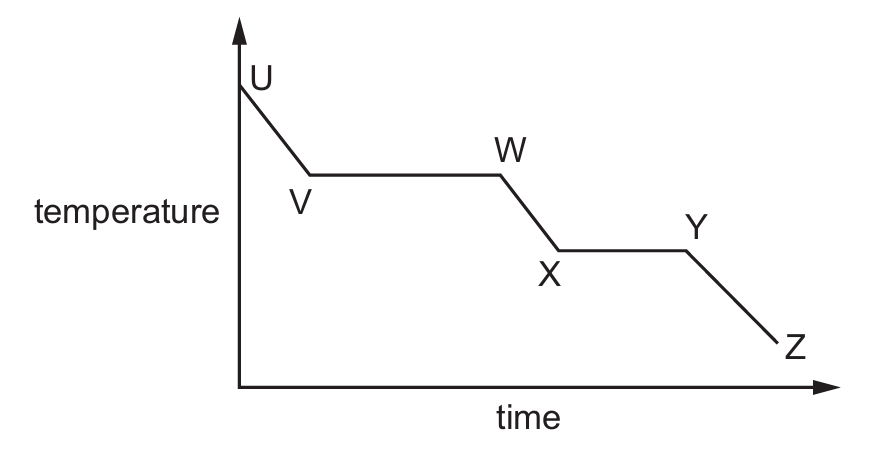
Which statement is correct?
A) Between U and V, the substance is condensing.
B) Between V and W, heat is being absorbed from the surroundings.
C) Between W and X, the particles are close together and randomly arranged.
D) Between Y and Z, the substance is changing from a liquid to a solid.
▶️ Answer/Explanation
Ans: C
On a cooling curve, the horizontal sections represent phase changes where temperature remains constant. Between W and X, the substance is in the liquid state where particles are close together but randomly arranged. Option A is incorrect because U-V shows cooling of gas, not condensation. B is incorrect because V-W shows gas-liquid transition where heat is being released, not absorbed. D is incorrect because Y-Z shows solid cooling, not liquid-solid transition.
Samples of four gases are released in a room at the same time.
The gases are carbon dioxide, CO2, hydrogen chloride, HCl, hydrogen sulfide, H2S, and nitrogen dioxide, NO2.
Which gas diffuses fastest?
A) carbon dioxide
B) hydrogen chloride
C) hydrogen sulfide
D) nitrogen dioxide
▶️ Answer/Explanation
Ans: C
The rate of diffusion is inversely proportional to the square root of the molar mass (Graham’s Law). Calculating molar masses: CO2 (44 g/mol), HCl (36.5 g/mol), H2S (34 g/mol), NO2 (46 g/mol). H2S has the lowest molar mass and therefore diffuses fastest. Even though HCl has a lower molecular weight than CO2, H2S is lighter than both.
Sulfur atoms can form the negative ion S2-.
Three other atoms or ions are listed.
- argon, Ar
- calcium, Ca
- oxide, O2-
How many of these atoms or ions have the same electronic configuration as S2-?
A) 0
B) 1
C) 2
D) 3
▶️ Answer/Explanation
Ans: B
Sulfur (atomic number 16) forms S2- by gaining 2 electrons, giving it 18 electrons – the same configuration as argon. Calcium (atomic number 20) loses 2 electrons to form Ca2+ with 18 electrons, but neutral Ca has 20 electrons. Oxide O2- has 10 electrons (8+2). Only argon (18 electrons) matches S2-‘s configuration. Therefore, only 1 (argon) has the same electronic configuration.
Element T has two isotopes, 126T and 146T.
Which statement about these isotopes is correct?
A) They have different chemical properties because they have different numbers of neutrons.
B) They have the same chemical properties because they have the same number of outer shell electrons.
C) They have the same nucleon number because the sum of the number of protons and electrons is the same.
D) They have different positions in the Periodic Table because they have different numbers of neutrons.
▶️ Answer/Explanation
Ans: B
Isotopes are atoms of the same element with different numbers of neutrons but the same number of protons and electrons. Chemical properties depend on electron configuration, so isotopes have identical chemical properties (same number of valence electrons). Option A is incorrect because chemical properties don’t depend on neutrons. C is incorrect because nucleon number is protons + neutrons (different for isotopes). D is incorrect because isotopes occupy the same position in the Periodic Table (same atomic number).
Lithium is in Group I of the Periodic Table. Nitrogen is in Group V of the Periodic Table.
Lithium reacts with nitrogen to form the ionic compound lithium nitride, Li3N.
What happens to the electrons when lithium atoms and nitrogen atoms form ions?
| lithium | nitrogen | |
|---|---|---|
| A) | each lithium atom loses one electron to form an Li+ ion | each nitrogen atom gains three electrons to form an N3- ion |
| B) | each lithium atom loses one electron to form an Li+ ion | each nitrogen atom gains five electrons to form an N5- ion |
| C) | each lithium atom gains one electron to form an Li– ion | each nitrogen atom loses three electrons to form an N3+ ion |
| D) | each lithium atom gains one electron to form an Li– ion | each nitrogen atom loses five electrons to form an N5+ ion |
▶️ Answer/Explanation
Ans: A
Lithium is in Group I, so it has 1 valence electron. To achieve stability, it loses this electron to form Li+ ion.
Nitrogen is in Group V with 5 valence electrons. It needs 3 more electrons to complete its octet, so it gains 3 electrons to form N3- ion.
The formula Li3N shows that 3 Li+ ions balance 1 N3- ion, confirming option A is correct.
For which covalent compound does the dot-and-cross diagram correctly show the outer shell electrons?
A) 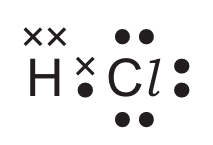
B) 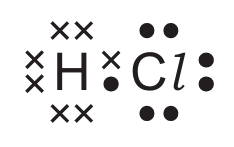
C) 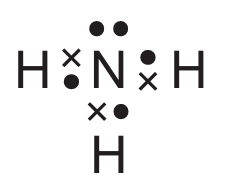
D) 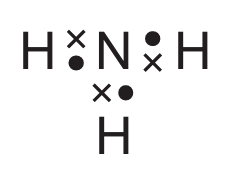
▶️ Answer/Explanation
Ans: C
Option C correctly shows the dot-and-cross diagram for ammonia (NH3):
- Nitrogen has 5 valence electrons (shown as dots/crosses)
- Each hydrogen contributes 1 electron (shown differently from nitrogen’s electrons)
- Three covalent bonds are formed (each pair of electrons between N and H)
- One lone pair remains on nitrogen
Which row identifies the positive and the negative particles present in a giant metallic lattice?
| positive particles | negative particles | |
|---|---|---|
| A) | anions | cations |
| B) | anions | delocalised electrons |
| C) | cations | anions |
| D) | cations | delocalised electrons |
▶️ Answer/Explanation
Ans: D
In a metallic lattice:
- The positive particles are cations (positively charged metal ions)
- The negative particles are delocalised electrons (electrons that are free to move throughout the structure)
Which formula for the named compound is correct?
A) calcium oxide, CaO
B) cobalt(II) chloride, Co2C1
C) sulfur dioxide, S2O2
D) anhydrous copper(II) sulfate, Cu(SO4)2
▶️ Answer/Explanation
Ans: A
Option A is correct because:
- Calcium (Ca2+) and oxide (O2-) ions combine in a 1:1 ratio to form CaO
- The charges balance perfectly (2+ and 2- cancel out)
Remember that for ionic compounds, the formula must reflect the simplest ratio of ions that balances the charges.
The equation for the reaction of magnesium with dilute sulfuric acid is shown.
Mg + H2SO4 → MgSO4 + H2
[Mr: MgSO4, 120]
Which mass of magnesium sulfate is formed when 12 g of magnesium completely reacts with dilute sulfuric acid?
A 5 g
B 10 g
C 60 g
D 120 g
▶️ Answer/Explanation
Ans: C
To solve this stoichiometry problem:
- First, find the moles of magnesium reacted:
- Molar mass of Mg = 24 g/mol
- Moles of Mg = mass/molar mass = 12 g / 24 g/mol = 0.5 mol
- From the balanced equation, the mole ratio of Mg:MgSO4 is 1:1
- So 0.5 mol of Mg will produce 0.5 mol of MgSO4
- Calculate the mass of MgSO4 produced:
- Molar mass of MgSO4 = 120 g/mol (given)
- Mass = moles × molar mass = 0.5 mol × 120 g/mol = 60 g
Therefore, the correct answer is C (60 g).
An organic compound, Q, contains carbon, hydrogen and oxygen only.
Q contains 40.0% carbon and 6.7% hydrogen by mass.
What is the empirical formula of Q?
A) CHO
B) CH2O
C) C2HO2
D) C3H6O3
▶️ Answer/Explanation
Ans: B
To find the empirical formula:
1. Assume 100g of the compound, so we have 40.0g C, 6.7g H, and the remainder is O (100 – 40.0 – 6.7 = 53.3g O).
2. Convert masses to moles:
– C: 40.0g ÷ 12g/mol ≈ 3.33 mol
– H: 6.7g ÷ 1g/mol = 6.7 mol
– O: 53.3g ÷ 16g/mol ≈ 3.33 mol
3. Divide each by the smallest number of moles (3.33):
– C: 3.33 ÷ 3.33 = 1
– H: 6.7 ÷ 3.33 ≈ 2
– O: 3.33 ÷ 3.33 = 1
4. This gives the ratio C1H2O1 or CH2O.
The value of the Avogadro constant is \(6.02 \times 10^{23}\).
What is the total number of atoms in 2.00 mol of ammonia gas?
A) \(1.20 \times 10^{24}\)
B) \(2.41 \times 10^{24}\)
C) \(4.82 \times 10^{24}\)
D) \(2.89 \times 10^{25}\)
▶️ Answer/Explanation
Ans: C
1. First, determine the number of molecules in 2.00 mol of ammonia:
\(2.00 \text{ mol} \times 6.02 \times 10^{23} \text{ molecules/mol} = 1.204 \times 10^{24} \text{ molecules}\)
2. Each ammonia molecule (NH3) contains 4 atoms (1 N + 3 H).
3. Total number of atoms:
\(1.204 \times 10^{24} \text{ molecules} \times 4 \text{ atoms/molecule} = 4.816 \times 10^{24} \text{ atoms}\)
4. This matches option C (\(4.82 \times 10^{24}\)) when rounded to 3 significant figures.
Three aqueous solutions, L, M and N, are electrolysed using inert electrodes.
L is concentrated hydrochloric acid.
M is concentrated aqueous sodium chloride.
N is dilute aqueous sodium chloride.
Which solutions produce a pale yellow-green gas at the anode?
A) L and M
B) L only
C) M and N
D) N only
▶️ Answer/Explanation
Ans: A
The pale yellow-green gas is chlorine (Cl2).
1. For L (concentrated HCl):
– At anode: 2Cl– → Cl2 + 2e–
– Chlorine gas is produced.
2. For M (concentrated NaCl):
– At anode: 2Cl– → Cl2 + 2e– (chloride ions are preferentially discharged over hydroxide ions in concentrated solution)
3. For N (dilute NaCl):
– At anode: 4OH– → 2H2O + O2 + 4e– (oxygen is produced instead of chlorine in dilute solution)
Therefore, only L and M produce chlorine gas at the anode.
Dilute sulfuric acid is electrolysed using inert electrodes.
What are the ionic half-equations for the reactions that take place at each electrode?
| positive electrode | negative electrode | |
|---|---|---|
| A | \(2H^+ + 2e^- \rightarrow H_2\) | \(40H^− \rightarrow 2H_2O + O_2 + 4e^-\) |
| B | \(2H^+ + 2e^- \rightarrow H_2\) | \(40H^− + 4H^+ \rightarrow 4H_2O\) |
| C | \(40H^− \rightarrow 2H_2O + O_2 + 4e^-\) | \(2H^+ + 2e^− \rightarrow H_2\) |
| D | \(40H^− + 4H^+ \rightarrow 4H_2O\) | \(2H^+ + 2e^− \rightarrow H_2\) |
▶️ Answer/Explanation
Ans: C
In the electrolysis of dilute sulfuric acid:
1. At the positive electrode (anode):
– OH– ions are discharged: \(4OH^− \rightarrow 2H_2O + O_2 + 4e^-\)
– Oxygen gas is produced
2. At the negative electrode (cathode):
– H+ ions are discharged: \(2H^+ + 2e^− \rightarrow H_2\)
– Hydrogen gas is produced
Note: The positive electrode is where oxidation occurs (loss of electrons), and the negative electrode is where reduction occurs (gain of electrons).
Which statements about hydrogen-oxygen fuel cells are correct?
- They convert chemical energy into electrical energy.
- Hydrogen is reduced in the fuel cells.
- They do not produce any atmospheric pollutants.
A) 1, 2 and 3
B) 1 and 2 only
C) 1 and 3 only
D) 2 and 3 only
▶️ Answer/Explanation
Ans: C
Let’s evaluate each statement:
1. Correct: Fuel cells convert the chemical energy of hydrogen and oxygen directly into electrical energy.
2. Incorrect: In fuel cells, hydrogen is oxidized (loses electrons) at the anode, not reduced. Oxygen is reduced at the cathode.
3. Correct: The only product of a hydrogen-oxygen fuel cell is water, making it pollution-free.
Therefore, only statements 1 and 3 are correct.
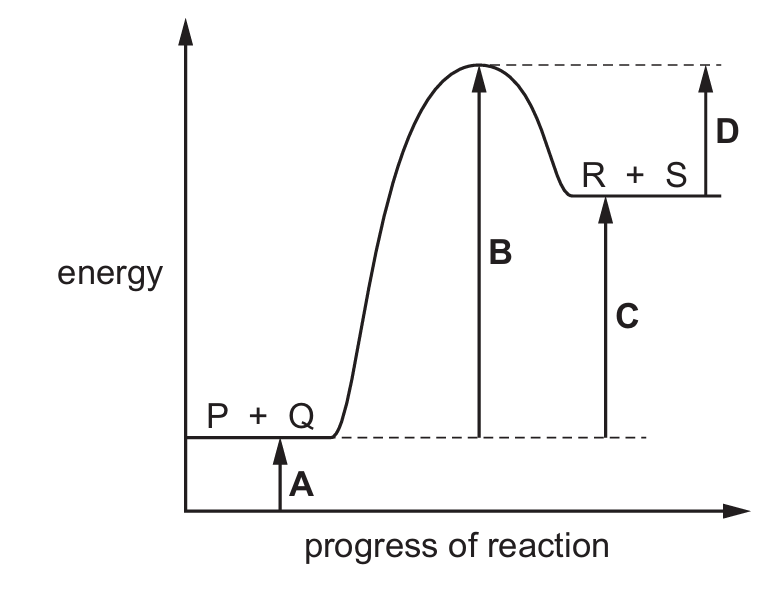
The reaction pathway diagram for the reaction between P and Q to form R and S is shown.
Which letter represents the enthalpy change for the reaction?
▶️ Answer/Explanation
Ans: C
The enthalpy change (ΔH) for a reaction is the difference in energy between the products and reactants. In a reaction pathway diagram, this is represented by the vertical distance between the energy levels of the reactants (P + Q) and products (R + S). The letter C correctly shows this difference, representing the enthalpy change of the reaction.
The equation for the complete combustion of methane is shown.
\[ \text{CH}_4(g) + 2\text{O}_2(g) \rightarrow \text{CO}_2(g) + 2\text{H}_2\text{O}(g) \]
The table shows some bond energies.
| bond | bond energy in kJ/mol |
|---|---|
| C–H | 410 |
| C=O | 805 |
| O=O | 496 |
| O–H | 460 |
What is the enthalpy change for this reaction?
A) -1458 kJ/mol
B) -818 kJ/mol
C) -359 kJ/mol
D) +102 kJ/mol
▶️ Answer/Explanation
Ans: B
To calculate the enthalpy change:
1. Bonds broken (endothermic, positive values):
– 4 C-H bonds: 4 × 410 = 1640 kJ/mol
– 2 O=O bonds: 2 × 496 = 992 kJ/mol
Total energy absorbed = 1640 + 992 = 2632 kJ/mol
2. Bonds formed (exothermic, negative values):
– 2 C=O bonds: 2 × 805 = 1610 kJ/mol
– 4 O-H bonds: 4 × 460 = 1840 kJ/mol
Total energy released = -(1610 + 1840) = -3450 kJ/mol
3. Enthalpy change:
ΔH = Energy absorbed + Energy released
ΔH = 2632 + (-3450) = -818 kJ/mol
The negative sign indicates the reaction is exothermic.
Which change is a physical change?
A) cracking an alkane
B) evaporating ethanol
C) fermenting glucose
D) neutralising an acid
▶️ Answer/Explanation
Ans: B
A physical change is one where the substance changes form but its chemical composition remains the same.
Option B (evaporating ethanol) is correct because it’s simply a phase change from liquid to gas with no change in molecular structure.
The other options are chemical changes:
– Cracking (A) breaks large hydrocarbon molecules into smaller ones
– Fermentation (C) converts glucose into ethanol and CO₂
– Neutralization (D) forms new products (salt and water)
Which statements explain why increasing the temperature in a reaction involving gases increases the rate of reaction?
- It increases the collision frequency between the gas particles.
- It lowers the activation energy.
- It increases the kinetic energy of the gas particles.
- It increases the number of gas particles per unit volume.
A) 1 and 3
B) 1 and 4
C) 2 and 3
D) 2 and 4
▶️ Answer/Explanation
Ans: A
Increasing temperature affects reaction rate through two main mechanisms:
1. Increases collision frequency: At higher temperatures, particles move faster, leading to more frequent collisions per second.
3. Increases kinetic energy: More particles have sufficient energy to overcome the activation energy barrier, increasing the proportion of successful collisions.
Statement 2 is incorrect because temperature doesn’t change the activation energy – it just gives more particles enough energy to overcome it. Statement 4 is incorrect because the number of particles per unit volume (concentration) remains constant unless the container size changes.
The equation for the reaction between ammonia and oxygen is shown.
\[ 4NH_3(g) + 5O_2(g) \rightleftharpoons 4NO(g) + 6H_2O(g) \quad \Delta H = -909 \, \text{kJ/mol} \]
Which two changes to the reaction conditions will both move the position of equilibrium to the right?
| change 1 | change 2 | |
|---|---|---|
| A | increasing the temperature | decreasing the pressure |
| B | increasing the temperature | increasing the pressure |
| C | decreasing the temperature | increasing the pressure |
| D | decreasing the temperature | decreasing the pressure |
▶️ Answer/Explanation
Ans: D
To shift equilibrium to the right (toward products), we apply Le Chatelier’s Principle:
Temperature: The reaction is exothermic (ΔH negative), so decreasing temperature favors the forward reaction (produces heat to counteract the change).
Pressure: There are 9 moles of gas on the left (4+5) and 10 on the right (4+6). Decreasing pressure favors the side with more moles of gas (products), moving equilibrium right.
Therefore, both decreasing temperature and decreasing pressure will shift equilibrium to the right (Option D).
Mercury(II) oxide, HgO, decomposes when heated.
The equation is shown.
\[ 2HgO \rightarrow 2Hg + O_2 \]
Why is this a reduction reaction?
A) The products weigh less than the reactants.
B) There are fewer reactants than products.
C) There is a gain of oxygen.
D) There is a loss of oxygen.
▶️ Answer/Explanation
Ans: D
This is a reduction reaction because mercury(II) oxide loses oxygen. In chemical terms:
1. Reduction is defined as the loss of oxygen from a compound.
2. In the reaction, HgO (mercury oxide) loses oxygen to form Hg (mercury).
3. The oxygen is released as O2 gas.
4. Option A is incorrect because mass conservation means products and reactants have equal mass.
5. Option B is irrelevant to the definition of reduction.
6. Option C describes oxidation, not reduction.
Some information about two dilute acids is shown.
| dilute acid | acid concentration in mol/dm3 | pH |
|---|---|---|
| nitric acid | 0.1 | 1.0 |
| propanoic acid | 0.4 | 2.6 |
Three statements about the acids are listed.
- Nitric acid has a lower pH because it dissociates more than propanoic acid.
- Propanoic acid has a lower concentration of hydrogen ions than nitric acid.
- Propanoic acid has a higher pH because it has a higher concentration.
Which statements are correct?
A) 1 and 2
B) 1 and 3
C) 2 only
D) 3 only
▶️ Answer/Explanation
Ans: A
Let’s analyze each statement:
Statement 1: Correct. Nitric acid is a strong acid that completely dissociates, while propanoic acid is a weak acid that only partially dissociates. This explains why nitric acid has a lower pH despite its lower concentration.
Statement 2: Correct. Even though propanoic acid has a higher concentration (0.4 mol/dm3 vs 0.1 mol/dm3), its partial dissociation means it produces fewer H+ ions than the completely dissociated nitric acid.
Statement 3: Incorrect. The higher pH of propanoic acid is due to its weaker acidity (less dissociation), not its higher concentration. In fact, if it were a strong acid, higher concentration would lead to lower pH.
Element E is a metal in Group I of the Periodic Table and element G is a non-metal in Group VII. Both of these elements form oxides.
Which statement about their oxides is correct?
A) Both oxides are acidic.
B) Both oxides are basic.
C) The oxide of E is acidic and the oxide of G is basic.
D) The oxide of G is acidic and the oxide of E is basic.
▶️ Answer/Explanation
Ans: D
Understanding the nature of oxides:
1. Group I metals (like E): Form basic oxides. For example, sodium oxide (Na2O) reacts with water to form alkaline solutions (NaOH).
2. Group VII non-metals (like G): Form acidic oxides. For example, chlorine oxides (Cl2O, ClO2) react with water to form acidic solutions (HClO, HClO2).
3. This is because metals tend to form ionic oxides that produce hydroxide ions in water (basic), while non-metals form covalent oxides that produce hydrogen ions in water (acidic).
4. Therefore, option D correctly describes the nature of both oxides.
Lead(II) sulfate is an insoluble salt.
Which method is suitable for obtaining pure solid lead(II) sulfate?
A) Mix aqueous lead(II) nitrate and aqueous potassium sulfate, heat to evaporate all of the water, collect the solid and then wash and dry it.
B) Mix aqueous lead(II) nitrate and aqueous potassium sulfate, filter, collect the filtrate, crystallise, then wash and dry the crystals.
C) Mix aqueous lead(II) nitrate and dilute sulfuric acid, filter, then wash and dry the residue.
D) Titrate aqueous lead(II) hydroxide with dilute sulfuric acid, crystallise, then wash and dry the crystals.
▶️ Answer/Explanation
Ans: C
For preparing insoluble salts like lead(II) sulfate:
1. Option A: Incorrect because heating to evaporate water would leave behind all dissolved salts (including soluble byproducts), not just the insoluble PbSO4.
2. Option B: Incorrect because collecting the filtrate would give you the soluble products, not the insoluble PbSO4 which would be in the residue.
3. Option C: Correct. The steps are:
- Mix solutions to form the insoluble salt: Pb(NO3)2 + H2SO4 → PbSO4↓ + 2HNO3
- Filter to collect the insoluble PbSO4 as residue
- Wash with distilled water to remove soluble impurities
- Dry to obtain pure PbSO4
4. Option D: Incorrect because titration is used for soluble salts, and lead(II) hydroxide is itself insoluble.
The elements oxygen and sulfur are in the same group of the Periodic Table.
Which statement about oxygen and sulfur is not correct?
A) They are non-metals.
B) They have giant covalent structures.
C) They have six electrons in the outer electron shells of their atoms.
D) They react together to form an acidic oxide.
▶️ Answer/Explanation
Ans: B
Analyzing each option:
1. Option A: Correct. Both oxygen and sulfur are non-metals in Group VI.
2. Option B: Incorrect (and thus the answer). Oxygen exists as O2 molecules (simple molecular structure), while sulfur exists as S8 molecules (also simple molecular) at room temperature. Neither has a giant covalent structure like diamond or silicon dioxide.
3. Option C: Correct. Both have 6 valence electrons (Group VI elements).
4. Option D: Correct. When they react (forming SO2 or SO3), these are acidic oxides that form acids when dissolved in water.
Therefore, statement B is the one that’s not correct.
Tennessine, Ts, is at the bottom of Group VII of the Periodic Table.
What are the predicted properties of tennessine at room temperature?
A) a black solid, more dense than iodine
B) a black solid, more reactive than iodine
C) a colourless gas, less dense than chlorine
D) a colourless gas, less reactive than chlorine
▶️ Answer/Explanation
Ans: A
Tennessine is in Group VII (halogens) and at the bottom of the group. We can predict its properties by looking at the trend in Group VII elements:
1. As we go down Group VII, the elements change from gases (F₂, Cl₂) to liquids (Br₂) to solids (I₂).
2. The density increases down the group (I₂ is a shiny black solid).
3. Reactivity decreases down the group (fluorine is most reactive, iodine less so).
Therefore, tennessine would be expected to be a black solid (like iodine but more dense) and less reactive than iodine. This matches option A.
An example of sacrificial protection is the fitting of zinc blocks to the outside of a ship’s steel hull.
Which statement explains why zinc is used to protect the iron in the steel from rusting?
A) Zinc is more reactive than iron so it loses electrons more easily.
B) Zinc is less reactive than iron so it loses electrons more easily.
C) Zinc is more reactive than iron so it gains electrons more easily.
D) Zinc is less reactive than iron so it gains electrons more easily.
▶️ Answer/Explanation
Ans: A
Sacrificial protection works by attaching a more reactive metal to the iron/steel structure. Here’s why:
1. Zinc is higher than iron in the reactivity series, meaning it’s more reactive.
2. In the presence of water and oxygen (conditions for rusting), zinc will oxidize (lose electrons) more readily than iron.
3. The zinc acts as the anode and corrodes instead of the iron, which becomes the cathode where reduction occurs.
4. This process continues until all the zinc is used up (sacrificed), hence the name “sacrificial protection”.
Option A correctly states that zinc is more reactive and loses electrons more easily than iron.
Which statement about alloys is correct?
A) Alloys are harder than pure metals because they contain strong intermolecular forces.
B) Brass is an alloy containing mainly copper and tin.
C) The different-sized atoms in an alloy mean that the layers cannot easily slide over each other.
D) There are no alloys containing carbon because carbon is a non-metal.
▶️ Answer/Explanation
Ans: C
Let’s analyze each option:
A) Incorrect – Alloys are harder due to disrupted metallic structure, not intermolecular forces.
B) Incorrect – Brass is copper and zinc, not tin (bronze is copper and tin).
C) Correct – The different sized atoms in alloys disrupt the regular layers, preventing them from sliding easily, making the alloy harder.
D) Incorrect – Steel is an alloy of iron and carbon, disproving this statement.
The correct explanation is that the irregular atomic sizes in alloys prevent the layers from sliding past each other, increasing hardness.
Separate pieces of aluminium foil and copper foil are heated in air.
The copper foil reacts to give a black solid.
The aluminium foil does not react.
Which statement explains these observations?
A) Aluminium has an unreactive layer, but copper does not.
B) Aluminium is below copper in the reactivity series.
C) Copper reacts with moisture in the air, but aluminium does not.
D) Copper reacts with nitrogen in the air, but aluminium does not.
▶️ Answer/Explanation
Ans: A
This question is about the reactivity of metals and their surface properties:
1. Aluminium is actually more reactive than copper in the reactivity series, which makes option B incorrect.
2. The reason aluminium doesn’t appear to react is that it quickly forms a thin, protective oxide layer (Al₂O₃) that prevents further reaction.
3. Copper doesn’t form such a protective layer, so it continues to react with oxygen in the air to form black copper(II) oxide (CuO).
4. Both metals would react with oxygen, not nitrogen (eliminating option D), and moisture isn’t the key factor here (eliminating option C).
Therefore, option A correctly explains the observations.
Which row gives the symbol equation for the formation of carbon monoxide and for the reduction of iron(III) oxide in a blast furnace?
| equation for the formation of carbon monoxide | equation for the reduction of iron(III) oxide | |
|---|---|---|
| A | 2C + O₂ → 2CO | FeO + CO → Fe + CO₂ |
| B | CO₂ + C → 2CO | FeO + CO → Fe + CO₂ |
| C | C + O₂ → CO₂ | Fe₂O₃ + 3CO → 2Fe + 3CO₂ |
| D | CO₂ + C → 2CO | Fe₂O₃ + 3CO → 2Fe + 3CO₂ |
▶️ Answer/Explanation
Ans: D
This question tests knowledge of blast furnace reactions:
Formation of carbon monoxide:
In the blast furnace, carbon dioxide reacts with carbon (coke) to form carbon monoxide:
CO₂ + C → 2CO
This eliminates options A and C.
Reduction of iron(III) oxide:
The iron ore used is hematite (Fe₂O₃), not FeO. The correct reduction equation is:
Fe₂O₃ + 3CO → 2Fe + 3CO₂
This eliminates options A and B.
Only option D has both correct equations – the Boudouard reaction for CO formation and the correct reduction of hematite.
A sample of river water contains a high concentration of nitrates from fertilisers.
Which statements about the river water are correct?
- It has a boiling point of 100°C.
- Its melting point is below 0°C.
- It turns anhydrous cobalt(II) chloride from pink to blue.
- It turns anhydrous copper(II) sulfate from white to blue.
A) 1 and 3
B) 1 and 4
C) 2 and 3
D) 2 and 4
▶️ Answer/Explanation
Ans: D
Let’s analyze each statement:
1. The boiling point of water with dissolved nitrates will be higher than 100°C due to boiling point elevation, so this is incorrect.
2. The melting point will indeed be below 0°C due to freezing point depression caused by the dissolved nitrates.
3. Anhydrous cobalt(II) chloride turns from blue to pink when hydrated, not the other way around.
4. Anhydrous copper(II) sulfate does turn from white to blue when hydrated, which would happen with water.
Therefore, only statements 2 and 4 are correct.
Which statements about the treatment of domestic water supplies are correct?
- The water undergoes sedimentation to remove dissolved solids.
- The water is filtered to remove insoluble solids.
- The water is treated with carbon to improve the taste.
- The water is chlorinated to decrease the pH.
A) 1 and 2
B) 1 and 4
C) 2 and 3
D) 3 and 4
▶️ Answer/Explanation
Ans: C
Let’s evaluate each statement:
1. Sedimentation removes suspended solids, not dissolved solids, so this is incorrect.
2. Filtration does remove insoluble solids, so this is correct.
3. Activated carbon is indeed used to improve taste by removing organic compounds, so this is correct.
4. Chlorination is for disinfection, not pH adjustment, so this is incorrect.
Therefore, only statements 2 and 3 are correct.
An experiment to find the percentage of oxygen in 150 cm³ of polluted air is shown.
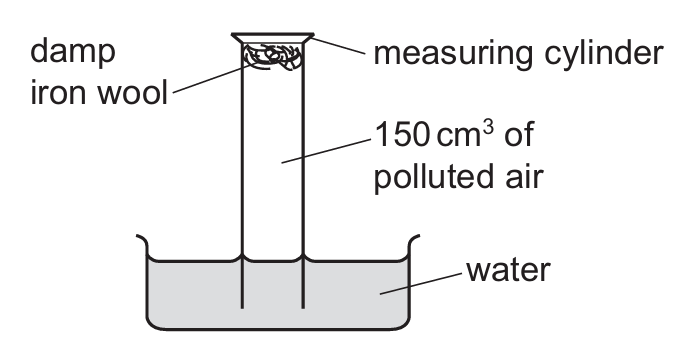
The apparatus is left for one week.
After this time, the volume of gas in the measuring cylinder is 122 cm³.
What is the percentage of oxygen, to the nearest whole number, in the polluted air?
A) 19%
B) 21%
C) 28%
D) 81%
▶️ Answer/Explanation
Ans: A
The damp iron wool reacts with the oxygen in the air to form rust (iron oxide). The volume decrease represents the oxygen that was present.
Initial volume = 150 cm³
Final volume = 122 cm³
Volume of oxygen reacted = 150 – 122 = 28 cm³
Percentage of oxygen = (28/150) × 100 ≈ 18.67%
Rounded to the nearest whole number, this is 19%.
Nitrogen monoxide, NO, and carbon monoxide, CO, are both removed from the exhaust gases of a car by a catalytic converter.
Which statement describes how nitrogen monoxide and carbon monoxide are removed by a catalytic converter?
A) Nitrogen monoxide and carbon monoxide are both reduced.
B) Nitrogen monoxide and carbon monoxide are both oxidised.
C) Nitrogen monoxide is oxidised and carbon monoxide is reduced.
D) Nitrogen monoxide is reduced and carbon monoxide is oxidised.
▶️ Answer/Explanation
Ans: D
In a catalytic converter:
1. Nitrogen monoxide (NO) is reduced to nitrogen (N₂):
2NO → N₂ + O₂ (reduction as nitrogen gains electrons)
2. Carbon monoxide (CO) is oxidized to carbon dioxide (CO₂):
2CO + O₂ → 2CO₂ (oxidation as carbon loses electrons)
Therefore, NO is reduced while CO is oxidized.
Propan-1-ol is oxidised by acidified aqueous potassium manganate(VII) in a similar way to ethanol.
Which compound is produced by the oxidation of propan-1-ol with acidified aqueous potassium manganate(VII)?
A) CH₃CH₂OH
B) CH₃CH₂CH₂OH
C) CH₃COOH
D) CH₃CH₂COOH
▶️ Answer/Explanation
Ans: D
Propan-1-ol (CH₃CH₂CH₂OH) undergoes oxidation with acidified potassium manganate(VII) as follows:
Primary alcohol → Aldehyde → Carboxylic acid
For propan-1-ol:
CH₃CH₂CH₂OH → CH₃CH₂CHO → CH₃CH₂COOH (propanoic acid)
The strong oxidizing conditions mean the reaction goes all the way to the carboxylic acid.
Option A is ethanol, B is propan-1-ol itself, C is ethanoic acid, and D is propanoic acid – the correct product.
The structural formula of methyl propane is CH3CH(CH3)CH3.
The equation represents the reaction of methyl propane with chlorine.
\[ C_4H_{10} + Cl_2 \rightarrow C_4H_9Cl + HCl \]
How many structural isomers with the molecular formula C4H9Cl can be formed from this reaction?
A) 1
B) 2
C) 3
D) 4
▶️ Answer/Explanation
Ans: B
Methyl propane (isobutane) has the structure CH3-CH(CH3)-CH3. When it reacts with chlorine, the chlorine can substitute a hydrogen atom at two different positions:
1. On the central carbon (CH3-C(Cl)(CH3)-CH3)
2. On any of the three equivalent methyl group carbons (CH2Cl-CH(CH3)-CH3)
These represent two distinct structural isomers. The first case gives 1-chloro-2-methylpropane, and the second case gives 2-chloro-2-methylpropane. Therefore, there are only 2 possible structural isomers.
Which statements describe disadvantages of manufacturing ethanol by fermentation?
- The process uses a renewable resource.
- The process produces impure ethanol.
- The process requires a high temperature.
- The process is slow.
A) 1 and 3
B) 1 and 4
C) 2 and 3
D) 2 and 4
▶️ Answer/Explanation
Ans: D
Let’s analyze each statement:
1. This is actually an advantage, not a disadvantage, as renewable resources are environmentally friendly.
2. Correct – fermentation produces a dilute solution of ethanol (about 15%) that requires further processing (distillation) to purify.
3. Incorrect – fermentation occurs at relatively low temperatures (typically 20-37°C).
4. Correct – fermentation is a biological process that takes several days to complete, much slower than industrial chemical processes.
Therefore, the correct disadvantages are statements 2 and 4.
Nylon is made in a polymerisation reaction.
Which row describes the type of polymerisation and identifies the other product of the reaction?
| type of polymerisation | other product | |
|---|---|---|
| A | addition | water |
| B | addition | none |
| C | condensation | water |
| D | condensation | none |
▶️ Answer/Explanation
Ans: C
Nylon is formed through condensation polymerization, where monomers join together with the elimination of small molecules (in this case, water).
In the formation of nylon:
– A diamine and a dicarboxylic acid react
– The -NH2 group of the diamine reacts with the -COOH group of the acid
– Water (H2O) is eliminated as a byproduct
– This forms an amide linkage (-CONH-) which repeats to form the polymer chain
Therefore, the correct description is condensation polymerization with water as the other product.
Which ion forms a green precipitate with aqueous sodium hydroxide that dissolves in an excess of aqueous sodium hydroxide?
A) Ca2+
B) Cr3+
C) Cu2+
D) Fe2+
▶️ Answer/Explanation
Ans: B
Let’s analyze each option:
A) Ca2+: Forms a white precipitate of Ca(OH)2 which doesn’t dissolve in excess NaOH.
B) Cr3+: Forms a green precipitate of Cr(OH)3 which dissolves in excess NaOH to form a green solution of [Cr(OH)4]–.
C) Cu2+: Forms a blue precipitate of Cu(OH)2 which doesn’t dissolve in excess NaOH.
D) Fe2+: Forms a green precipitate of Fe(OH)2 which doesn’t dissolve in excess NaOH (and turns brown as it oxidizes to Fe(OH)3).
Only chromium(III) ions (Cr3+) match all the given conditions: green precipitate that dissolves in excess NaOH.
A mixture of soluble substances can be separated by paper chromatography. Each substance can be identified using its Rf value.
Which formula shows how the Rf value is calculated?
A) Rf = distance travelled by solvent / distance travelled by substance
B) Rf = distance travelled by substance / distance travelled by solvent
C) Rf = distance travelled by solvent – distance travelled by substance
D) Rf = distance travelled by solvent × distance travelled by substance
▶️ Answer/Explanation
Ans: B
The Rf (retention factor) value is calculated as:
\[ R_f = \frac{\text{distance travelled by substance}}{\text{distance travelled by solvent}} \]
Key points about Rf values:
1. The distance is measured from the origin (starting point) to the center of the spot.
2. The solvent front is marked when the chromatography is stopped.
3. Rf values are always between 0 and 1.
4. Each substance has a characteristic Rf value under specific conditions (same solvent, paper type, temperature).
The correct formula is option B, where the distance travelled by the substance is divided by the distance travelled by the solvent.
