A list of symbols and formulae is shown.
\(\begin{matrix}
Ba^{2+}\\
C \\
Cl_{2} \\
Cl^{-} \\
CO \\
CO_{2} \\
CuO \\
H_{2} \\
H_{2}O \\
K^{+} \\
Na^{+} \\
O^{2-} \\
\end{matrix}\)
Answer the following questions using only these symbols and formulae. Each symbol or formula may be used once, more than once or not at all.
(a) State which symbol or formula represents:
(i) an element used as an inert electrode for electrolysis
(ii) an ion formed when an atom gains two electrons
(iii) a basic oxide
(iv) an ion that gives a yellow colour in a flame test
(v) a toxic gas formed during the incomplete combustion of methane
(vi) an element used as a reactant in a fuel cell.
(b) Water, \( H_2O \), is a simple covalent molecule.
Complete Fig. 1.1 to show the dot-and-cross diagram for a molecule of water. Show outer shell electrons only.
▶️ Answer/Explanation
(a)
(i) C (Carbon) – Carbon is often used as an inert electrode in electrolysis because it is conductive and doesn’t react with most electrolytes.
(ii) \( O^{2-} \) – When an oxygen atom gains two electrons, it forms an oxide ion with a 2- charge.
(iii) CuO (Copper oxide) – Copper oxide is a basic oxide that reacts with acids to form salts and water.
(iv) \( Na^+ \) – Sodium ions give a characteristic yellow color in flame tests due to the excitation of electrons in the sodium atoms.
(v) CO (Carbon monoxide) – This toxic gas is produced during incomplete combustion of methane when there’s insufficient oxygen.
(vi) \( H_2 \) (Hydrogen) – Hydrogen is used as a reactant in fuel cells where it combines with oxygen to produce electricity and water.
(b) The dot-and-cross diagram for water should show:
- Two hydrogen atoms each sharing one electron with the oxygen atom (covalent bonds)
- The oxygen atom should have two lone pairs (four non-bonding electrons)
- Each hydrogen atom should have no other electrons (just the one shared with oxygen)
The correct diagram would show: H (with one electron) sharing with O (with six valence electrons, two of which are shared with H atoms), resulting in two single covalent bonds and two lone pairs on oxygen.
(a) Table 2.1 shows information about the reactions of four different metals with oxygen.
| metal | reaction with oxygen |
|---|---|
| calcium | burns rapidly to form an oxide |
| cobalt | burns slowly to form an oxide |
| platinum | no reaction |
| tin | forms an oxide but does not burn |
Put the four metals in order of their reactivity. Put the most reactive metal first.
(b) Cobalt is a transition element. Lithium is an element in Group I of the Periodic Table.
Describe two differences in the physical properties of cobalt compared to lithium.
(c) Brass and stainless steel are alloys.
(i) Describe what is meant by the term alloy.
(ii) Name the two metals in brass.
(iii) Give one use for stainless steel.
(d) (i) Deduce the number of protons, neutrons and electrons in the cobalt ion shown.
\( ^{59}_{27}Co^{3+} \)
(ii) State the charge on a neutron.
▶️ Answer/Explanation
(a) calcium > cobalt > tin > platinum
Calcium is the most reactive as it burns rapidly with oxygen. Cobalt comes next as it burns slowly. Tin forms an oxide but doesn’t burn, showing less reactivity. Platinum shows no reaction, making it the least reactive.
(b) Any two of:
1. Cobalt has a high melting point/boiling point while lithium has a low melting point/boiling point
2. Cobalt has a high density while lithium has a low density
3. Cobalt is strong/hard while lithium is weak/soft
Transition metals like cobalt typically have higher melting points and densities compared to Group 1 metals like lithium.
(c)(i) An alloy is a mixture of a metal with another element or another metal.
Alloys are created to enhance properties like strength, durability, or resistance to corrosion.
(c)(ii) The two metals in brass are copper and zinc.
Brass is an alloy typically composed of copper (about 60-70%) and zinc (about 30-40%).
(c)(iii) One use for stainless steel is cutlery.
Stainless steel is widely used in cutlery because of its corrosion resistance and durability.
(d)(i)
Number of protons: 27 (same as atomic number)
Number of neutrons: 33
Number of electrons: 24 (27 protons minus 3+ charge)
For the cobalt ion \( ^{59}_{27}Co^{3+} \), we subtract 3 electrons from the neutral atom’s 27 electrons.
(d)(ii) The charge on a neutron is 0 (zero).
Neutrons are neutral subatomic particles with no electric charge.
A student investigates the reaction of magnesium with dilute sulfuric acid.
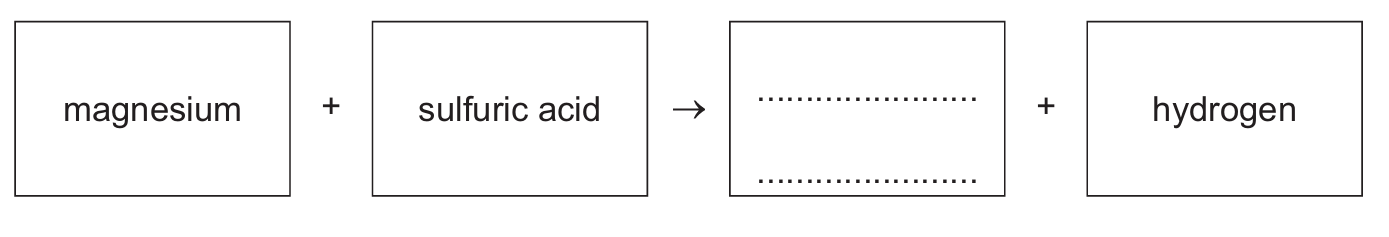
(a) Complete the word equation for the reaction of magnesium with dilute sulfuric acid.
(b) The student sets up an experiment to investigate the rate of this chemical reaction.
The student:
- carries out the experiment at 25°C using 2.00 g of magnesium ribbon and 25 cm³ of dilute sulfuric acid
- measures the total volume of gas produced at regular time intervals
- plots a graph of the results.
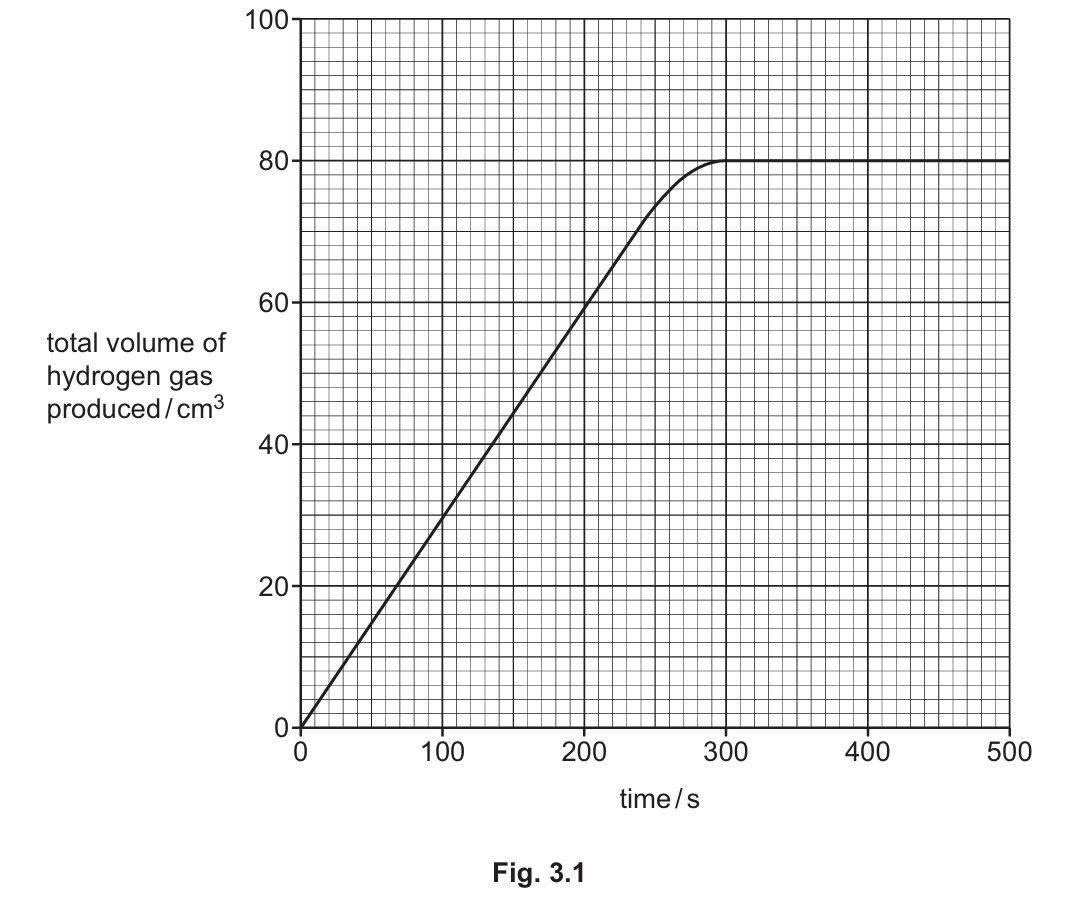
Fig. 3.1 shows the graph of the student’s results.
(i) Draw a labelled diagram of the apparatus the student should use to carry out this investigation.
(ii) Deduce the total volume of hydrogen gas produced in the first 100 s of this reaction.
(iii) Suggest why the reaction stops at 300 s.
(iv) The experiment is repeated at a higher temperature. All other conditions stay the same. Draw a line on the grid in Fig. 3.1 to show how the volume of hydrogen gas produced changes at a higher temperature.
(v) The student repeats the experiment using sulfuric acid of a lower concentration. All other conditions stay the same. Describe how the rate of the reaction differs when sulfuric acid of a lower concentration is used.
(vi) The student repeats the experiment using a catalyst. All other conditions stay the same. Describe how the rate of the reaction differs when a catalyst is used.
(c) Describe a test for hydrogen.
▶️ Answer/Explanation
(a) magnesium + sulfuric acid → magnesium sulfate + hydrogen
The reaction between magnesium and sulfuric acid is a single displacement reaction where magnesium replaces hydrogen in the acid, forming magnesium sulfate and releasing hydrogen gas.
(b)(i) The apparatus should include:
- A conical flask containing the magnesium ribbon and sulfuric acid
- A gas syringe connected to the flask to measure the volume of hydrogen gas produced
- Proper sealing to ensure no gas escapes
(b)(ii) 30 cm³
From the graph, at 100 seconds, the volume of hydrogen gas produced is approximately 30 cm³.
(b)(iii) The reaction stops because one of the reactants has been completely used up (either all the magnesium has reacted or all the sulfuric acid has been neutralized).
(b)(iv) The line should:
- Start at the same point (0,0)
- Have a steeper initial gradient (faster reaction rate at higher temperature)
- Level off before 300 seconds (reaction completes faster)
- Reach the same final volume (100 cm³) as the same amount of reactants were used
(b)(v) The rate of reaction decreases (gets slower) when using sulfuric acid of lower concentration because there are fewer acid particles per unit volume, resulting in fewer successful collisions between reactant particles per unit time.
(b)(vi) The rate of reaction increases (gets faster) when using a catalyst because the catalyst provides an alternative reaction pathway with lower activation energy, allowing more successful collisions between reactant particles.
(c) Test: Insert a lighted splint into the gas.
Observation: The gas burns with a ‘pop’ sound (explosive reaction with oxygen).
This is the characteristic test for hydrogen gas, as hydrogen is highly flammable and reacts explosively with oxygen when ignited.
(a) Fig. 4.1 shows the displayed formula of compound B.
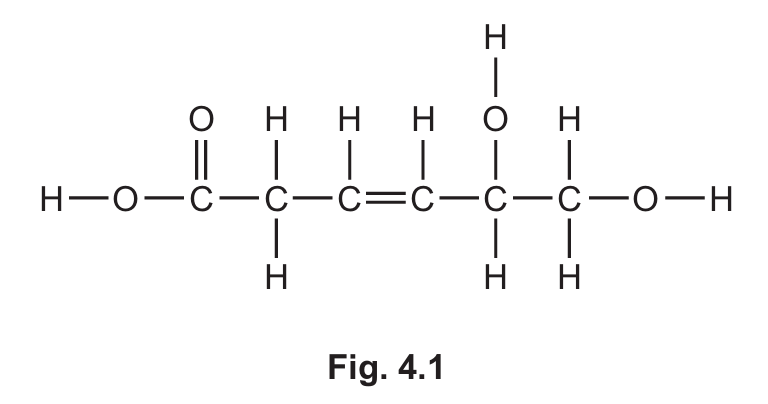
(i) On Fig. 4.1, draw a circle around the carboxylic acid functional group.
(ii) Deduce the molecular formula of compound B.
(iii) Explain why compound B is unsaturated.
(iv) Describe a test for an unsaturated compound.
(b) Alkanes are hydrocarbons.
(i) State the type of bonding in an alkane molecule.
(ii) Ethane reacts with chlorine in a substitution reaction. Draw the displayed formula of the organic product of this reaction.
(iii) State the meaning of the term hydrocarbon.
(c) Petroleum contains hydrocarbons.
Name the process used to separate petroleum into its useful components.
(d) Complete Table 4.1 to show the name and use for some of the components in petroleum.
| name | use |
|---|---|
| gasoline/petrol | ………….. |
| ………….. | jet fuel |
| bitumen | ………….. |
▶️ Answer/Explanation
(a)(i) Circle around COOH group
The carboxylic acid functional group is -COOH, which should be circled in the structure.
(a)(ii) \( \text{C}_6\text{H}_{10}\text{O}_4 \)
Counting all atoms in the molecule: 6 carbon atoms, 10 hydrogen atoms, and 4 oxygen atoms.
(a)(iii) Has a C=C bond / has a carbon-carbon double bond
Unsaturated compounds contain double or triple bonds between carbon atoms. The presence of C=C makes it unsaturated.
(a)(iv) Test: aqueous bromine (1)
Observations: turns colorless / decolorizes (1)
Bromine water (orange/brown) reacts with double bonds, causing the solution to become colorless as bromine adds across the double bond.
(b)(i) covalent
Alkanes have single covalent bonds between carbon atoms and between carbon and hydrogen atoms.
(b)(ii) Displayed formula of chloroethane (CH3CH2Cl)
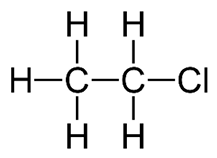
In the substitution reaction, one hydrogen in ethane is replaced by a chlorine atom.
(b)(iii) Compounds made up of carbon and hydrogen (atoms) only
Hydrocarbons are organic compounds consisting exclusively of hydrogen and carbon atoms.
(c) fractional distillation
Petroleum is separated into its components based on their different boiling points through fractional distillation.
(d) Table completion:
- gasoline/petrol: fuel for cars (1)
- kerosene / paraffin: jet fuel (1)
- bitumen: (making) roads (1)
These are common uses for different fractions obtained from petroleum distillation.
This question is about iron and its compounds.
(a) Fig. 5.1 shows the changes of the physical states of iron.
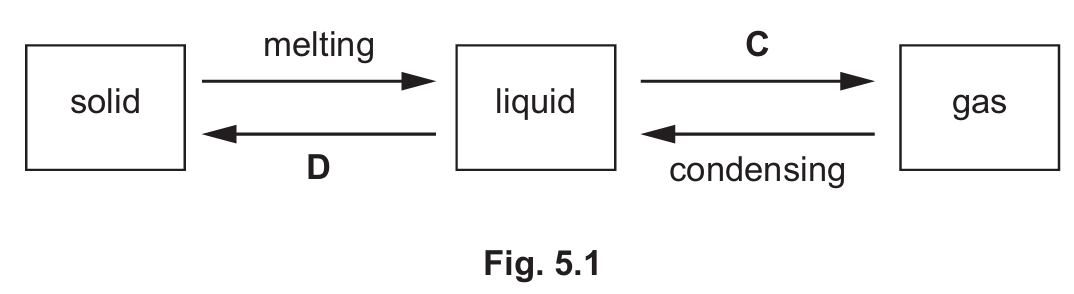
Name the changes of physical states C and D.
(b) Use the kinetic particle model to describe the arrangement and motion of the particles in liquid iron.
(c) Complete the symbol equation for the reaction of iron with oxygen.
\(….Fe + ….O_2 \rightarrow Fe_3O_4\)
(d) Iron can be obtained from iron ore in the blast furnace.
(i) Name the main ore of iron used in the blast furnace.
(ii) State one reason why carbon is burned in the blast furnace.
(iii) Name the solid compound that forms when limestone undergoes thermal decomposition.
(e) Iron is used as a catalyst in the reaction shown.
\(N_2 + 3H_2 \rightleftharpoons 2NH_3\)
State the meaning of the symbol \(\rightleftharpoons\).
(f) Iron rusts when in contact with air and water.
Name one barrier method used to prevent iron from rusting.
▶️ Answer/Explanation
(a) C – evaporation/boiling, D – freezing
The diagram shows the state changes of iron. C represents the transition from liquid to gas (evaporation or boiling), while D represents the transition from liquid to solid (freezing).
(b) arrangement: irregular/no particular arrangement
motion: sliding over each other/random
In the liquid state, iron particles are close together but not in a fixed arrangement. They can move past one another (slide) in a random fashion, which gives liquids their ability to flow.
(c) \(3Fe + 2O_2 \rightarrow Fe_3O_4\)
Balancing the equation requires 3 iron atoms on the left to match the 3 on the right (in Fe3O4). The oxygen atoms balance with 2 O2 molecules (4 oxygen atoms) on the left matching the 4 oxygen atoms in Fe3O4.
(d) (i) hematite
(ii) to provide heat/produce carbon dioxide
(iii) calcium oxide
Hematite (Fe2O3) is the main iron ore. Carbon burns to provide the high temperatures needed for the reduction process and also produces CO2 which reacts further to form CO, the reducing agent. Limestone (CaCO3) decomposes to calcium oxide (CaO) which helps remove impurities.
(e) reversible reaction
The double arrow symbol indicates the reaction can proceed in both directions – nitrogen and hydrogen can combine to form ammonia, while ammonia can also decompose back into nitrogen and hydrogen.
(f) painting/greasing/oiling/coating with plastic
Barrier methods prevent contact between iron and water/oxygen. Painting is the most common method, creating a physical barrier. Greasing/oiling are used for moving parts, while plastic coatings are used for pipes and other industrial applications.
This question is about acids and bases.
(a) Dilute hydrochloric acid reacts with calcium carbonate powder. Name the three products formed in this reaction.
(b) State the colour of methyl orange when it is added to dilute hydrochloric acid.
(c) For the test for halide ions, a dilute acid and aqueous silver nitrate are required.
(i) Explain why the acid used must not be hydrochloric acid.
(ii) Suggest a suitable dilute acid that can be used for the test for halide ions.
(d) Barium carbonate is insoluble in water. Choose from the list one other compound that is insoluble in water.
Tick (✓) one box.
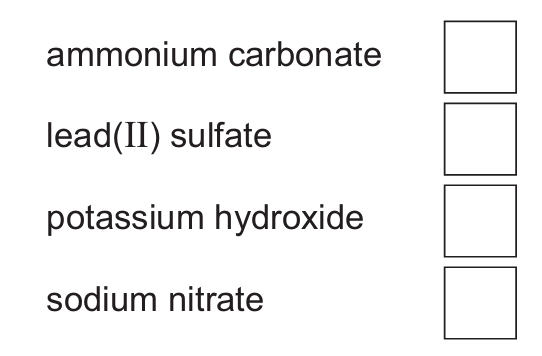
(e) Write the formula of the carbonate ion.
▶️ Answer/Explanation
(a) calcium chloride (1), water (1), carbon dioxide (1)
The reaction between hydrochloric acid and calcium carbonate is: \( \text{CaCO}_3 + 2\text{HCl} \rightarrow \text{CaCl}_2 + \text{H}_2\text{O} + \text{CO}_2 \). The products are calcium chloride (a salt), water, and carbon dioxide gas.
(b) red
Methyl orange is an indicator that turns red in acidic solutions (pH < 3.1) and yellow in basic solutions (pH > 4.4). Since hydrochloric acid is a strong acid, methyl orange will show its acidic color.
(c)(i) (hydrochloric acid contains a) halide / chloride / \( \text{Cl}^- \)
Hydrochloric acid contains chloride ions, which would give a false positive result in the halide test (white precipitate with silver nitrate).
(c)(ii) nitric acid
Nitric acid is suitable because it doesn’t contain halide ions and provides the acidic conditions needed for the test.
(d) second box down ticked (lead sulfate)
Lead(II) sulfate is insoluble in water, while the other options (ammonium carbonate, potassium hydroxide, sodium nitrate) are all soluble compounds.
(e) \( \text{CO}_3^{2-} \)
The carbonate ion has a formula of \( \text{CO}_3^{2-} \), consisting of one carbon atom and three oxygen atoms with a 2- charge.
(a) A list of common air and water pollutants is shown.
methane
nitrates
oxides of nitrogen
particulates
plastics
sewage
Answer the following questions using only these pollutants.
Each pollutant may be used once, more than once or not at all.
State which pollutant:
(i) contains harmful microbes that can cause disease
(ii) can be produced by the incomplete combustion of carbon-containing fuels
(iii) is a gas formed from the decomposition of vegetation.
(b) A 250 cm3 sample of polluted water contains 3.5 mg of particulates.
Calculate the mass of particulates present in 100 cm3 of this polluted water.
(c) Sulfur dioxide in the atmosphere causes acid rain.
State one method of reducing the emissions of sulfur dioxide to the atmosphere.
(d) Sulfur dioxide is a simple molecular compound.
State two physical properties of simple molecular compounds.
▶️ Answer/Explanation
(a)(i) sewage
Sewage contains harmful bacteria and other microbes that can cause diseases like cholera and dysentery when it contaminates water sources.
(a)(ii) particulates
Particulates (tiny solid particles) are produced during incomplete combustion of carbon-containing fuels like coal, diesel, or wood.
(a)(iii) methane
Methane is a natural gas produced when vegetation decomposes anaerobically (without oxygen), such as in swamps or landfills.
(b) 1.4 mg
Calculation: Using proportion – if 250 cm3 contains 3.5 mg, then 100 cm3 contains (3.5 × 100)/250 = 1.4 mg.
(c) low sulfur fuels / flue gas desulfurisation
Using fuels with lower sulfur content reduces SO2 emissions. Flue gas desulfurization (scrubbers) in power plants removes SO2 from exhaust gases.
(d) Any two of:
1. Low melting point / low boiling point
2. Poor electrical conductivity / poor thermal conductivity
Simple molecular compounds have weak intermolecular forces, leading to low melting/boiling points. They don’t conduct electricity as they have no free electrons or ions.
(a) Molten potassium chloride is electrolysed using inert electrodes.
(i) Name the product formed at each electrode.
product at positive electrode ……
product at negative electrode ……
(ii) State the general name of the negative electrode used in electrolysis.
(b) Potassium forms a salt when added to ethanoic acid.
Name the salt formed.
(c) Dilute ethanoic acid gives a yellow colour when tested with universal indicator.
Choose from the list the pH value for dilute ethanoic acid.
Draw a circle around your chosen answer.
pH1 pH5 pH7 pH13
(d) Ethanoic acid has the formula \( CH_3COOH \).
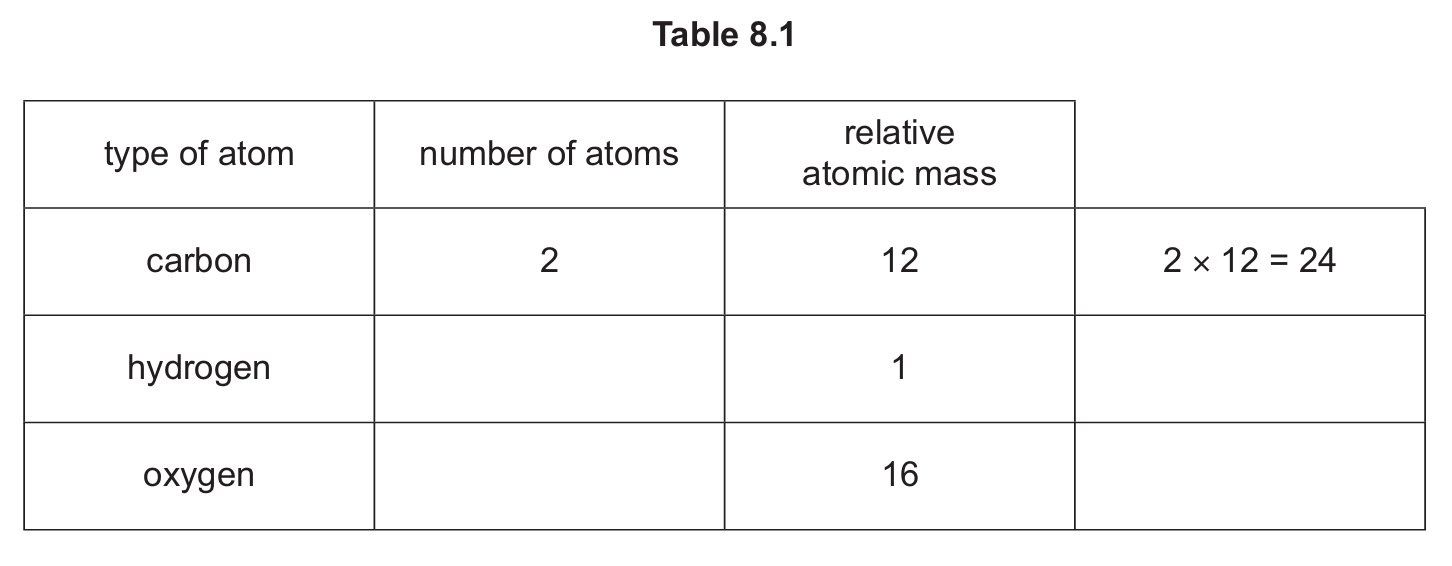
Complete Table 8.1 to calculate the relative molecular mass of \( CH_3COOH \).
(e) A molecule of ethanol also contains two carbon atoms.
(i) Ethanol can be manufactured by the addition of steam to ethene.
In this process, a pressure of 60 atm is used.
State two other conditions used in this process.
(ii) State one use of ethanol.
▶️ Answer/Explanation
(a)(i) positive electrode: chlorine / \( Cl_2 \) (1)
negative electrode: potassium / K (1)
During electrolysis of molten potassium chloride (\( KCl \)), potassium ions (\( K^+ \)) are reduced at the cathode (negative electrode) to form potassium metal, while chloride ions (\( Cl^- \)) are oxidized at the anode (positive electrode) to form chlorine gas.
(a)(ii) cathode (1)
The negative electrode in electrolysis is always called the cathode, where reduction occurs.
(b) potassium ethanoate (1)
When potassium reacts with ethanoic acid (\( CH_3COOH \)), it forms potassium ethanoate (\( CH_3COOK \)) and hydrogen gas.
(c) pH 5 (1)
Ethanoic acid is a weak acid, so its pH is higher than strong acids but still acidic. Yellow on universal indicator typically corresponds to pH 5.
(d) 60 (2)
If 2 marks are not scored: 1 mark for H = (4 × 1) = 4 or O = (2 × 16) = 32
Calculation:
Carbon: 2 atoms × 12 = 24
Hydrogen: 4 atoms × 1 = 4
Oxygen: 2 atoms × 16 = 32
Total = 24 + 4 + 32 = 60
(e)(i) high temperature / 300 °C (1)
acid catalyst (1)
The industrial production of ethanol from ethene requires high temperature (around 300°C) and an acid catalyst (typically phosphoric acid) to facilitate the reaction between ethene and steam.
(e)(ii) solvent / fuel (1)
Ethanol has multiple uses including as a solvent in various industries, as a fuel (either pure or in gasoline blends), and in alcoholic beverages.
