Using numbers only, state the:
(a) percentage of oxygen in clean, dry air …… .
(b) typical operating temperature, in °C, used in the Haber process …… .
(c) number of atoms in a diatomic molecule …… .
(d) maximum number of electrons in the second electron shell of an atom …… .
(e) number of hydrogen atoms in an alkane with 7 carbon atoms …… .
(f) number of particles in one mole, in standard form. …… .
▶️ Answer/Explanation
(a) 21
Clean, dry air contains approximately 21% oxygen by volume.
(b) 450
The Haber process, which synthesizes ammonia from nitrogen and hydrogen, typically operates at around 450°C.
(c) 2
A diatomic molecule consists of two atoms chemically bonded together (e.g., O₂, N₂, H₂).
(d) 8
The second electron shell can hold a maximum of 8 electrons according to the 2n² rule (where n=2: 2×2²=8).
(e) 16
Alkanes follow the general formula CₙH₂ₙ₊₂. For 7 carbon atoms: 2×7 + 2 = 16 hydrogen atoms.
(f) \(6.02 \times 10^{23}\)
One mole of any substance contains Avogadro’s number of particles, which is \(6.02 \times 10^{23}\) in standard form.
This question is about ionic compounds.
(a) State what is meant by the term ionic bond.
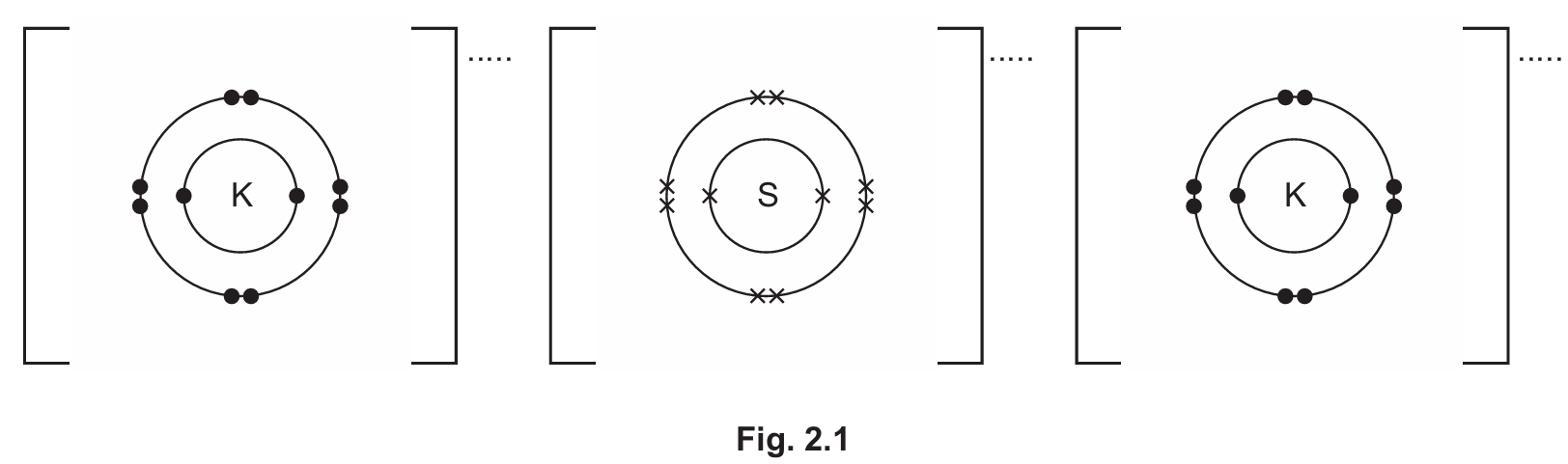
(b) Potassium sulfide, \( K_2S \), is an ionic compound. Complete the dot-and-cross diagram in Fig. 2.1 of the ions in potassium sulfide. Show the charges on the ions.
(c) Ionic compounds form giant ionic lattices.
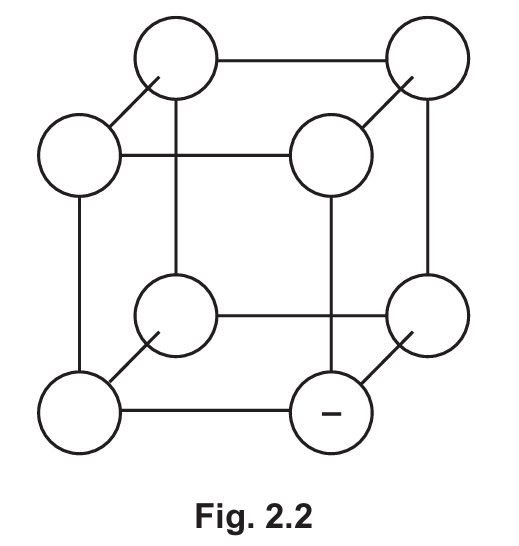
(i) Fig. 2.2 shows part of the giant ionic lattice structure of sodium chloride. Complete the diagram in Fig. 2.2 to show the ions present. Use ‘+’ for sodium ions and ‘-‘ for chloride ions. One chloride ion has been completed for you.
(ii) State the name given to any positive ion.
(d) Ionic compounds can be decomposed by the passage of an electric current using inert electrodes.
(i) State the name of this process.
(ii) Write the ionic half-equation for the reaction which takes place at the anode when molten potassium bromide, KBr, is decomposed by the passage of an electric current.
(iii) Name the products and state the observations at the negative and positive electrodes when dilute aqueous potassium bromide, KBr, is decomposed by the passage of an electric current.
▶️ Answer/Explanation
(a) An ionic bond is the strong electrostatic attraction between oppositely charged ions.
Detailed explanation: Ionic bonds form when electrons are transferred from metal atoms to non-metal atoms, creating positively charged cations and negatively charged anions that are strongly attracted to each other.
(b) The dot-and-cross diagram should show:
- Potassium ions (K⁺) with 8 electrons in their outer shell
- Sulfide ion (S²⁻) with 8 electrons in its outer shell (6 from sulfur and 2 gained)
- Correct charges shown (+ on K ions and 2- on S ion)
(c)(i) The diagram should show alternating positive (+) and negative (-) ions in a regular pattern, with each Na⁺ surrounded by Cl⁻ and vice versa.
(c)(ii) Cation
Explanation: Positive ions are called cations because they are attracted to the cathode during electrolysis.
(d)(i) Electrolysis
(d)(ii) \( 2Br^- \rightarrow Br_2 + 2e^- \)
Explanation: At the anode (positive electrode), bromide ions lose electrons to form bromine molecules. This is an oxidation half-equation.
(d)(iii)
- Product at negative electrode: Hydrogen
- Observation at negative electrode: Bubbles of gas
- Products at positive electrode: Oxygen and water
- Observation at positive electrode: Bubbles of gas
Detailed explanation: In dilute aqueous KBr, water molecules are preferentially discharged at the electrodes. At the cathode (negative electrode), hydrogen ions from water are reduced to hydrogen gas. At the anode (positive electrode), hydroxide ions from water are oxidized to oxygen gas and water.
The halogens are a group of elements in the Periodic Table. Chlorine is a member of this group.
(a) State the group number of the halogens.
(b) State how many halogens there are in this group.
(c) Suggest the identity of the halogen which:
(i) has the highest density
(ii) is the most reactive.
(d) State the name of the negative ions (anions) formed by halogens.
(e) State how many occupied electron shells there are in a bromine atom.
(f) Name the noble gas which has the same electronic configuration as a Br⁻ ion.
(g) Aqueous chlorine, \( \text{Cl}_2 \), reacts with aqueous potassium iodide, KI. One of the products formed is iodine, \( \text{I}_2 \).
(i) Complete and balance the ionic equation for the reaction between \( \text{Cl}_2 \) and \( \text{I}^- \) ions. State symbols are not required.
\[ \text{Cl}_2 + ….. \text{I}^- \rightarrow …… + \text{I}_2 \]
(ii) Explain why this reaction is defined as a redox reaction. Give your answer in terms of electron transfer.
(h) Give the colour and state of iodine at room temperature and pressure.
▶️ Answer/Explanation
(a) VII
The halogens are in Group VII (or Group 17 in modern IUPAC numbering) of the Periodic Table.
(b) 6
There are 6 halogens: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts).
(c)(i) tennessine / Ts / Ts₂
Tennessine, being the heaviest halogen, has the highest density as density increases down the group.
(c)(ii) fluorine / F / F₂
Fluorine is the most reactive halogen as reactivity decreases down the group due to decreasing electronegativity and increasing atomic size.
(d) halide(s)
The negative ions formed by halogens are called halides (e.g., chloride Cl⁻, bromide Br⁻, iodide I⁻).
(e) 4
Bromine (atomic number 35) has electron configuration 2,8,18,7, meaning it has 4 occupied electron shells.
(f) krypton
A Br⁻ ion has 36 electrons (35+1), which is the same electron configuration as krypton (Kr), the noble gas with atomic number 36.
(g)(i) \( \text{Cl}_2 + 2\text{I}^- \rightarrow 2\text{Cl}^- + \text{I}_2 \)
Chlorine displaces iodide ions from solution, forming chloride ions and iodine. The equation is balanced with 2 iodide ions producing 1 iodine molecule.
(g)(ii) I⁻ loses e⁻ (oxidation) and Cl₂ gains e⁻ (reduction)
This is a redox reaction because:
- Iodide ions (I⁻) lose electrons (oxidation) to form iodine (I₂)
- Chlorine molecules (Cl₂) gain electrons (reduction) to form chloride ions (Cl⁻)
The electron transfer is evident as chlorine oxidizes iodide ions.
(h) colour: grey-black, state: solid
At room temperature and pressure, iodine exists as grey-black solid crystals with a metallic luster.
Carbonyl chloride, COCl2, is manufactured by reacting carbon monoxide with chlorine.
\[ \text{CO(g)} + \text{Cl}_2(\text{g}) \rightleftharpoons \text{COCl}_2(\text{g}) \]
\[ \Delta H = -105 \, \text{kJ/mol} \]
The process takes place in a closed system, and an equilibrium is reached. The conditions for this process are 200°C and 200 kPa.
(a) Explain what is meant by the term closed system.
(b) State what the symbol \(\Delta H\) represents.
(c) State how the value of \(\Delta H\) shows that the forward reaction is exothermic.
(d) Deduce the value of \(\Delta H\) for the reverse reaction. Include a sign in your answer.
(e) Complete Table 4.1 to show the effect, if any, on the concentration of \(\text{COCl}_2(\text{g})\) at equilibrium when the following changes to the conditions are applied.
Use only the words increases, decreases or no change.
| change to conditions | effect on the concentration of \(\text{COCl}_2(\text{g})\) at equilibrium |
|---|---|
| the temperature is increased | |
| some CO is added | |
| the pressure is increased | |
| a catalyst is added |
(f) The equation for the reaction can be represented as shown in Fig. 4.1.
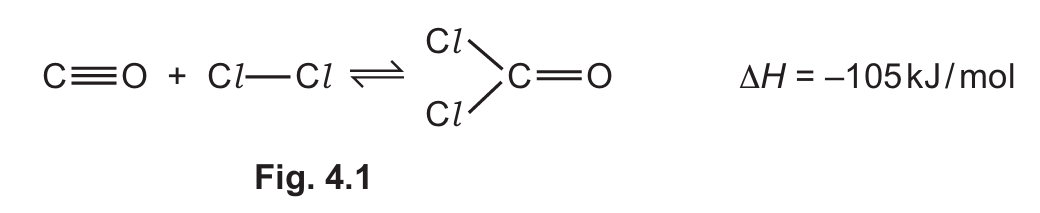
Table 4.2 shows some bond energies.
| bond | C≡O | Cl-Cl | C-Cl |
|---|---|---|---|
| bond energy in kJ/mol | 1075 | 240 | 340 |
Use the bond energies in Table 4.2 and the value of \(\Delta H\) for the reaction to calculate the bond energy, in kJ/mol, of the C=O bond.
Use the following steps.
- Calculate the energy needed to break the bonds in the reactants.
- Calculate the energy released when the bonds in carbonyl chloride form.
- Calculate the bond energy of the C=O bond.
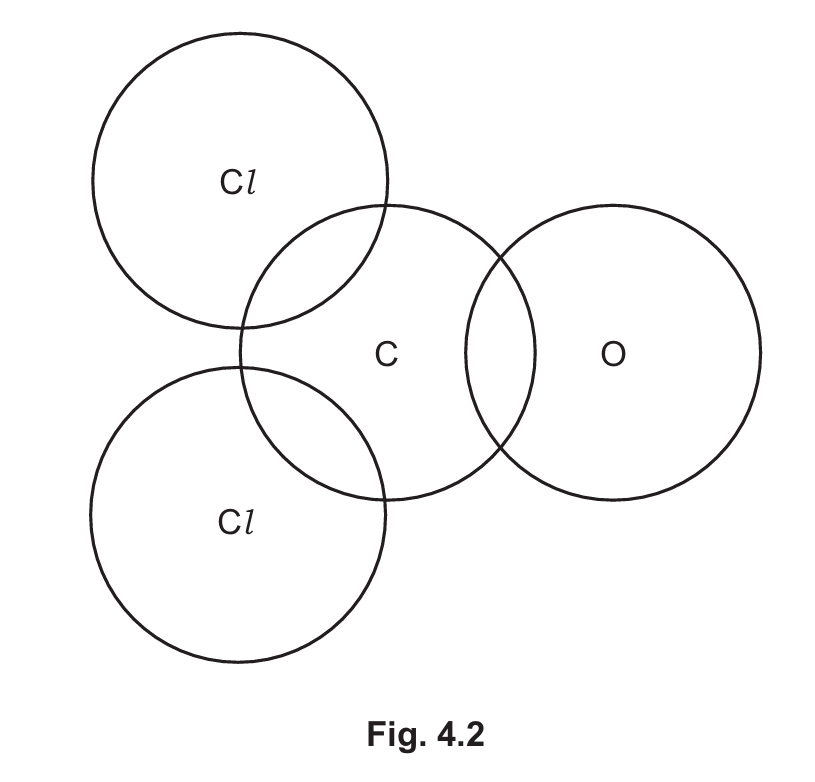
(g) Complete the dot-and-cross diagram in Fig. 4.2 of a molecule of carbonyl chloride.
Show outer shell electrons only.
▶️ Answer/Explanation
(a) A closed system is one where no reactants or products can enter or leave, though energy can be exchanged with the surroundings.
(b) \(\Delta H\) represents the enthalpy change of the reaction, which is the heat energy change at constant pressure.
(c) The negative value of \(\Delta H\) (-105 kJ/mol) shows that the forward reaction is exothermic because energy is released to the surroundings.
(d) The reverse reaction would have \(\Delta H = +105 \, \text{kJ/mol}\) because it’s the exact opposite of the forward reaction (endothermic).
(e) Table 4.1 completed:
| the temperature is increased | decreases |
| some CO is added | increases |
| the pressure is increased | increases |
| a catalyst is added | no change |
Explanation: Increasing temperature favors the endothermic direction (reverse reaction). Adding CO shifts equilibrium right. Increasing pressure favors side with fewer gas molecules (product side). Catalyst affects rate but not position of equilibrium.
(f) Calculation steps:
1. Energy to break bonds in reactants = C≡O + Cl-Cl = 1075 + 240 = 1315 kJ
2. Energy released when bonds form in product = 1315 – (-105) = 1420 kJ
3. Let C=O bond energy be x. Then for COCl2: x + 2(C-Cl) = 1420 → x + 2(340) = 1420 → x = 1420 – 680 = 740 kJ/mol
Final answer: The bond energy of C=O is 740 kJ/mol.
(g) Dot-and-cross diagram for COCl2:
Cl: with 6 outer electrons (dots/crosses) and one shared pair with C
C: with 4 outer electrons, sharing single bonds with both Cl atoms and a double bond with O
O: with 6 outer electrons, sharing a double bond with C
Each bond should show either dots or crosses to represent shared electrons from each atom.
Manganese is the element with atomic number 25 in the Periodic Table.
Calcium is the element with atomic number 20 in the Periodic Table.
(a) Complete Table 5.1 to show the number of protons, neutrons and electrons in the \(^{55}\)Mn atom and the \(^{42}\)Ca\(^{2+}\) ion.
| \(^{55}\)Mn | \(^{42}\)Ca\(^{2+}\) | |
|---|---|---|
| protons | ||
| neutrons | ||
| electrons |
(b) Manganese forms several oxides. The formulae of some of these oxides are shown.
MnO
Mn\(_2\)O\(_3\)
Mn\(_3\)O\(_4\)
MnO\(_2\)
Mn\(_2\)O\(_7\)
(i) Suggest why manganese is expected to form coloured oxides.
(ii) State which other property of manganese is shown by the formation of several oxides.
(iii) State the formula of manganese(II) oxide.
(c) Mn\(_3\)O\(_4\) is found in an ore of manganese. Manganese metal can be extracted from Mn\(_3\)O\(_4\) using aluminium as the reducing agent.
(i) Define the term reducing agent.
(ii) Complete the symbol equation by inserting the formula of the missing product and balancing the equation.
\[ \text{….} \, \text{Mn}_3\text{O}_4 + …. \, \text{Al} \rightarrow \text{……} + …. \, \text{Mn} \]
(d) MnO\(_2\) reacts with dilute hydrochloric acid as shown in the equation.
\[ \text{MnO}_2 + 4\text{HCl} \rightarrow \text{MnCl}_2 + 2\text{H}_2\text{O} + \text{Cl}_2 \]
(i) Calculate the volume of chlorine gas formed, in cm\(^3\), at r.t.p. when excess MnO\(_2\) reacts with 50.0 cm\(^3\) of 0.200 mol/dm\(^3\) HCl.
(ii) Describe a test for chlorine gas.
(iii) Explain, in terms of collision theory, why decreasing the temperature decreases the rate of this reaction.
▶️ Answer/Explanation
(a)
| \(^{55}\)Mn | \(^{42}\)Ca\(^{2+}\) | |
|---|---|---|
| protons | 25 | 20 |
| neutrons | 30 | 22 |
| electrons | 25 | 18 |
For \(^{55}\)Mn: protons = atomic number = 25; neutrons = mass number – protons = 55-25 = 30; electrons = protons = 25 (neutral atom).
For \(^{42}\)Ca\(^{2+}\): protons = atomic number = 20; neutrons = 42-20 = 22; electrons = 20-2 = 18 (2+ ion).
(b)(i) (Mn is a) transition element
Transition metal compounds are often colored due to the presence of partially filled d-orbitals which allow electron transitions that absorb visible light.
(b)(ii) variable oxidation state
The different oxides show manganese in different oxidation states (+2 in MnO, +3 in Mn\(_2\)O\(_3\), etc.), demonstrating variable oxidation states.
(b)(iii) MnO
The (II) indicates manganese has a +2 oxidation state in this oxide.
(c)(i) a substance that reduces another substance and is itself oxidised
A reducing agent donates electrons to another substance, causing that substance to be reduced while the reducing agent itself is oxidized.
(c)(ii) \[ 3\text{Mn}_3\text{O}_4 + 8\text{Al} \rightarrow 4\text{Al}_2\text{O}_3 + 9\text{Mn} \]
The thermite reaction produces aluminum oxide and manganese metal. Balancing: 3 Mn\(_3\)O\(_4\) provides 9 Mn atoms, requiring 8 Al to form 4 Al\(_2\)O\(_3\).
(d)(i) 60.0 cm\(^3\)
Steps:
1. Moles HCl = 50.0 cm\(^3\) × 0.200 mol/dm\(^3\) ÷ 1000 = 0.0100 mol
2. From equation, 4 HCl produce 1 Cl\(_2\), so moles Cl\(_2\) = 0.0100 ÷ 4 = 0.00250 mol
3. Volume at r.t.p. = 0.00250 mol × 24000 cm\(^3\)/mol = 60.0 cm\(^3\)
(d)(ii) (damp) litmus (paper) and is bleached/goes white
Chlorine is a powerful oxidizing agent that bleaches the color from litmus paper.
(d)(iii) 1. Kinetic energy of particles decreases
2. Frequency of collisions between particles decreases
3. Lower percentage/proportion/fraction of collisions/particles have energy greater than/equal to activation energy
At lower temperatures, particles move slower with less energy, resulting in fewer effective collisions that can overcome the activation energy barrier.
The structural formulae of two compounds, A and B, are shown.
| A | B |
|---|---|
| CH2=CHCH3 | CH3CH=CHCH3 |
A and B are members of the same homologous series.
(a) Give two reasons why the structural formulae of A and B show they are members of the same homologous series.
(b) Explain why A and B are both hydrocarbons.
(c) Write the symbol equation for the complete combustion of A.
(d) Deduce the empirical formula of A.
(e) Name compound B.
(f) A structural isomer of B is a member of the same homologous series. Draw the displayed formula of this structural isomer of B.
(g) Compound B reacts with aqueous bromine at room temperature to form product C. The equation is shown.
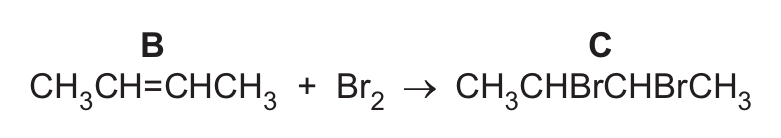
(i) State why this is an addition reaction.
(ii) Describe the colour change in aqueous bromine during this reaction.
(iii) Name product C.
(h) Under certain conditions, one mole of B reacts with oxygen to form two moles of carboxylic acid D. Carboxylic acid D has two carbon atoms.
(i) Draw the displayed formula of carboxylic acid D.
(ii) Name carboxylic acid D.
(iii) Complete the symbol equation for this reaction.
![]()
▶️ Answer/Explanation
(a) 1. Both have a general formula of CnH2n
2. Both have a C=C functional group (alkenes)
(b) Both compounds are made of carbon and hydrogen only, with no other elements present.
(c) 2CH2=CHCH3 + 9O2 → 6CO2 + 6H2O
For complete combustion of propene (C3H6), the products are always CO2 and H2O. Balancing the equation requires 2 molecules of propene reacting with 9 molecules of oxygen to produce 6 molecules each of carbon dioxide and water.
(d) CH2
The empirical formula is the simplest ratio of atoms in the compound. For propene (C3H6), dividing by 3 gives CH2.
(e) but-2-ene
This is a 4-carbon alkene with the double bond between the second and third carbons.
(f) The displayed formula of but-1-ene: CH2=CH-CH2-CH3
This is a structural isomer where the double bond is between the first and second carbons instead.
(g)(i) Only one product is formed from the combination of two reactants.
(g)(ii) Orange to colorless
The orange bromine solution becomes colorless as it reacts with the alkene.
(g)(iii) 2,3-dibromobutane
The product has bromine atoms added to carbons 2 and 3 of the butane chain.
(h)(i) The displayed formula of ethanoic acid: CH3COOH
(h)(ii) ethanoic acid
(h)(iii) CH3CH=CHCH3 + 2O2 → 2CH3COOH
This oxidative cleavage reaction breaks the double bond and adds oxygen to form two molecules of ethanoic acid.
