Question
Nitrogen is heated in a balloon, which expands slightly.
Which statements about the molecules of nitrogen are correct?
1 They move further apart.
2 They move more quickly.
3 They remain the same distance apart.
4 Their speed remains unchanged.
A 1 and 2
B 1 and 4
C 2 and 3
D 3 and 4
▶️Answer/Explanation
Ans:A
Question
Which piece of apparatus should be used to measure exactly $21.4 \mathrm{~cm}^3$ of water?
A $25 \mathrm{~cm}^3$ beaker
B $25 \mathrm{~cm}^3$ pipette
C $50 \mathrm{~cm}^3$ burette
D $50 \mathrm{~cm}^3$ measuring cylinder
▶️Answer/Explanation
Ans:C
Question
Which method of separation is used to separate a soluble solid from its solution?
A chromatography
B condensation
C crystallisation
D filtration
▶️Answer/Explanation
Ans:C
Question
The atomic number and nucleon number of a potassium atom are shown.
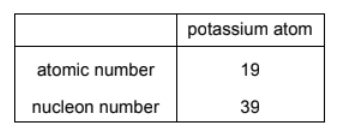
How many protons, neutrons and electrons are in a potassium ion, $\mathrm{K}^{+}$?

▶️Answer/Explanation
Ans:A
Question
Sodium is in Group I of the Periodic Table.
Chlorine is in Group VII of the Periodic Table.
Sodium and chlorine combine to form a compound.
Which statement about the combination of sodium and chlorine atoms is correct?
A Both sodium and chlorine lose electrons.
B Both sodium and chlorine gain electrons.
C Sodium loses electrons and chlorine gains electrons.
D Sodium gains electrons and chlorine loses electrons.
▶️Answer/Explanation
Ans:C
Question
The electronic structures of two atoms, $\mathrm{P}$ and $\mathrm{Q}$, are shown.
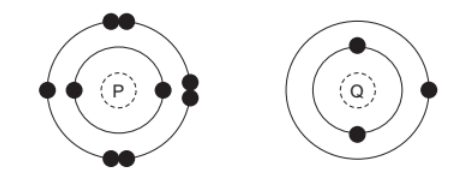
P and Q combine together to form a compound.
What is the type of bonding in the compound and what is the formula of the compound?

▶️Answer/Explanation
Ans:A
Question
The structures of diamond and graphite are shown.

Which statement about diamond and graphite is correct?
A Diamond and graphite have low melting points.
B Diamond and graphite have mobile electrons.
C Diamond and graphite have layered structures.
D Diamond and graphite contain strong covalent bonds between carbon atoms.
▶️Answer/Explanation
Ans:D
Question
Aluminium oxide has the formula $\mathrm{Al}_2 \mathrm{O}_3$.
Which statement about aluminium oxide is correct?
A $2 \mathrm{~g}$ of aluminium atoms are combined with $3 \mathrm{~g}$ of oxygen atoms.
B $2 \mathrm{~g}$ of aluminium atoms are combined with $3 \mathrm{~g}$ of oxygen molecules.
C Aluminium oxide has a relative formula mass of 102 .
D Pure aluminium oxide contains a higher mass of oxygen than of aluminium.
▶️Answer/Explanation
Ans:C
Question
Which products are formed when dilute sulfuric acid undergoes electrolysis?

▶️Answer/Explanation
Ans:A
Question
Which element is not used as a fuel?
A carbon
B helium
C hydrogen
D uranium
▶️Answer/Explanation
Ans:B
Question
The energy level diagram shows the energy of the reactants and products in a chemical reaction.

Which row correctly describes the energy change and the type of reaction shown?

▶️Answer/Explanation
Ans:B
Question
Which diagram represents a chemical change?

▶️Answer/Explanation
Ans:D
Question
The rate of reaction between magnesium and hydrochloric acid is investigated. The volume of hydrogen given off at different times is measured. The results are shown.

Which conclusions are correct?
1 The rate is fastest between 0 and 20 seconds.
2 The maximum volume of hydrogen given off is $22 \mathrm{~cm}^3$.
3 At 40 seconds, $20 \mathrm{~cm}^3$ of hydrogen is given off.
A 1 and 2 only
B 1 and 3 only
C 2 and 3 only
D 1,2 and 3
▶️Answer/Explanation
Ans:A
Question
Which reaction can be easily reversed?
A dissolving zinc in hydrochloric acid
B fermenting glucose with yeast
C heating hydrated cobalt(II) chloride
D the rusting of an iron nail
▶️Answer/Explanation
Ans:C
Question
Carbon reacts with silver oxide to form carbon dioxide and silver.
Which substance is reduced?
A carbon
B carbon dioxide
C silver
D silver oxide
▶️Answer/Explanation
Ans:D
Question
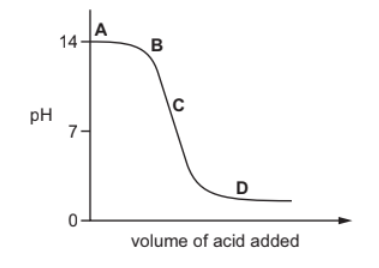
The graph shows how the $\mathrm{pH}$ of a solution changes as an acid is added to an alkali.
$
\text { acid }+ \text { alkali } \rightarrow \text { salt }+ \text { water }
$
Which letter represents the area of the graph where both acid and salt are present?
▶️Answer/Explanation
Ans:D
Question
Phosphorus is an element in Group V of the Periodic Table. It burns in air to form an oxide, which dissolves in water to form a solution with a pH of 1. Which row describes this oxide of phosphorus?

▶️Answer/Explanation
Ans:C
Question
The apparatus shown is used to prepare aqueous copper(II) sulfate.

What are X and Y?

▶️Answer/Explanation
Ans:C
Question
Two tests are carried out on substance $Z$.
test 1 A flame test produces a red flame.
test $2 \mathrm{Z}$ is dissolved in water and dilute nitric acid is added, followed by aqueous silver nitrate. A yellow precipitate is produced.
What is substance $\mathrm{Z}$ ?
A lithium bromide
B lithium iodide
C sodium bromide
D sodium iodide
▶️Answer/Explanation
Ans:B
Question
The elements in Period 3 of the Periodic Table are shown.

Which statements about the elements in Period 3 are correct?
$1 \mathrm{Na}, \mathrm{Mg}$ and $\mathrm{Al}$ are metals.
$2 \mathrm{~S}, \mathrm{Cl}$ and $\mathrm{Ar}$ are non-metals.
$3 \mathrm{Si}, \mathrm{P}$ and $\mathrm{S}$ are metals.
A 1 and 2 only
B 1 and 3 only
C 2 and 3 only
D 1,2 and 3
▶️Answer/Explanation
Ans:A
Question
A Group I metal (lithium, sodium or potassium) is reacted with a Group VII element (chlorine, bromine or iodine).
Which compound is formed when the Group I metal of highest density reacts with the Group VII element of lowest density?
A lithium chloride
B potassium chloride
C potassium iodide
D lithium iodide
▶️Answer/Explanation
Ans:B
Question
The properties of the element titanium, Ti, can be predicted from its position in the Periodic Table. Which row identifies the properties of titanium?

▶️Answer/Explanation
Ans:B
Question
A balloon is filled with helium. Helium is a noble gas and makes the balloon rise up in the air.
The density of air is $1.23 \mathrm{~g} / \mathrm{dm}^3$.
Which gas is helium?

▶️Answer/Explanation
Ans:B
Question
Which property is shown by all metals?
A They are extracted from their ores by heating with carbon.
B They conduct electricity.
C They form acidic oxides.
D They react with hydrochloric acid to form hydrogen.
▶️Answer/Explanation
Ans:C
Question
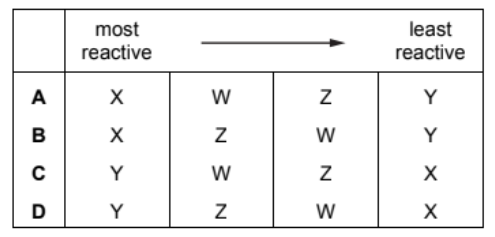
The properties of four metals, $\mathrm{W}, \mathrm{X}, \mathrm{Y}$ and $\mathrm{Z}$, are shown.
W It does not react with cold water but reacts with steam.
$X$ It does not react with water or dilute acid but the oxide of $X$ is reduced by carbon.
$Y$ The oxide of $Y$ is not reduced by carbon but $Y$ reacts vigorously with cold water.
Z It does not react with water or steam but reacts with dilute acid.
What is the order of reactivity of the elements starting with the most reactive?
▶️Answer/Explanation
Ans:C
Question
Molten iron from the blast furnace contains impurities.
The process of turning the impure iron into steel involves blowing oxygen into the molten iron and adding calcium oxide.
What are the reasons for blowing in oxygen and adding calcium oxide?

▶️Answer/Explanation
Ans:A
Question
Which row describes two uses of the named steel?

▶️Answer/Explanation
Ans:C
Question
Which statement shows that a liquid is pure water?
A It boils at $100^{\circ} \mathrm{C}$.
B It has a pH value of 7 .
C It turns blue cobalt(II) chloride pink.
D It turns white copper(II) sulfate blue.
▶️Answer/Explanation
Ans:A
Question
Some gases are present in clean air while other gases are only present in polluted air. Which row is correct?

▶️Answer/Explanation
Ans:B
Question
The diagrams show experiments to investigate rusting of iron nails.

In which test-tubes do the nails rust?
A 1 only
B 1 and 2 only
C 1 and 3 only
D 1,2 and 3
▶️Answer/Explanation
Ans:B
Question
Which mixture contains all of the elements in a typical fertiliser?
A ammonium nitrate and calcium phosphate
B ammonium phosphate and potassium chloride
C potassium nitrate and ammonium chloride
D potassium carbonate and ammonium nitrate
▶️Answer/Explanation
Ans:B
Question
Which processes produce methane?
1 complete combustion of carbon-containing compounds
2 decomposition of vegetation
3 digestion in animals
4 respiration in animals
A 1 and 4
B 1 and 3
C 2 and 3
D 2 and 4
▶️Answer/Explanation
Ans:C
Question
The list shows four methods that were suggested for the formation of carbon dioxide.
1 cracking methane using steam
2 action of heat on a carbonate
3 complete combustion of methane
4 reaction of a carbonate with oxygen
Which methods would result in the production of carbon dioxide?
A 1 and 2
B 1 and 4
C 2 and 3
D 3 and 4
▶️Answer/Explanation
Ans:C
Question
A student suggests three uses of calcium carbonate (limestone).
1 manufacture of cement
2 manufacture of iron
3 treating alkaline soils
Which suggestions are correct?
A 1 and 2 only
B 1 and 3 only
C 2 and 3 only
D 1,2 and 3
▶️Answer/Explanation
Ans:A
Question
Which list shows the fractions obtained from distilling petroleum, in order of increasing boiling point?
A bitumen $\rightarrow$ diesel oil $\rightarrow$ fuel oil $\rightarrow$ lubricating oil
B diesel oil $\rightarrow$ gasoline $\rightarrow$ naphtha $\rightarrow$ kerosene
C gasoline $\rightarrow$ naphtha $\rightarrow$ kerosene $\rightarrow$ diesel oil
D kerosene $\rightarrow$ lubricating oil $\rightarrow$ naphtha $\rightarrow$ refinery gas
▶️Answer/Explanation
Ans:C
Question
Which statement about members of a homologous series is correct?
A They are elements with the same chemical properties.
B They are compounds with the same functional group.
C They are atoms with the same number of outer electrons.
D They are molecules with the same boiling point.
▶️Answer/Explanation
Ans:B
Question
Increasing the number of atoms in one molecule of a hydrocarbon increases the amount of
energy released when it burns.
What is the correct order?

▶️Answer/Explanation
Ans:D
Question
Which statements about ethanol are correct?
1 Ethanol is made by reacting steam with ethene at $300^{\circ} \mathrm{C}$.
2 Ethanol is made by fermentation at $55^{\circ} \mathrm{C}$.
3 Ethanol burns to produce carbon dioxide and water.
4 Ethanol contains a carbon-carbon double bond.
A 1 and 2
B 1 and 3
C 2 and 3
D 3 and 4
▶️Answer/Explanation
Ans:B
Question
Some properties of an organic compound $\mathrm{J}$ are listed.
- It is a liquid at room temperature.
- It is soluble in water.
- A solution of $\mathrm{J}$ reacts with calcium carbonate to form carbon dioxide.
- A solution of $\mathrm{J}$ has a $\mathrm{pH}$ of 3 .
In which homologous series does $\mathrm{J}$ belong?
A alkane
B alkene
C alcohol
D carboxylic acid
▶️Answer/Explanation
Ans:D
Question
Which polymers or types of polymer are synthetic?
1 carbohydrates
2 nylon
3 proteins
4 Terylene
A 1 and 3
B 1 and 4
C 2 and 3
D 2 and 4
▶️Answer/Explanation
Ans:D
