Question
Which row describes the arrangement and movement of particles in a liquid?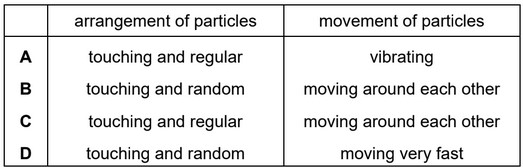
Answer/Explanation
Ans: B
Question
A mixture is separated using the apparatus shown.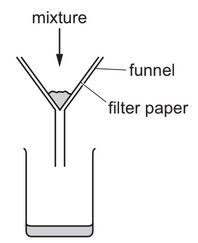
What is the mixture?
A aqueous copper(II) sulfate and aqueous sodium chloride
B aqueous copper(II) sulfate and copper
C copper and sulfur
D ethanol and ethanoic acid
Answer/Explanation
Ans: B
Question
Which statement about paper chromatography is correct?
A A solvent is needed to dissolve the paper.
B Paper chromatography separates mixtures of solvents.
C The solvent should cover the baseline.
D The baseline should be drawn in pencil.
Answer/Explanation
Ans: D
Question
Element X has 7 protons.
Element Y has 8 more protons than X.
Which statement about element Y is correct?
A Y has more electron shells than X.
B Y has more electrons in its outer shell than X.
C Y is in a different group of the Periodic Table from X.
D Y is in the same period of the Periodic Table as X.
Answer/Explanation
Ans: A
Question
A covalent molecule Q contains only six shared electrons.
What is Q?
A ammonia, \(NH_3\)
B chlorine, \(Cl_2\)
C methane, \(CH_4\)
D water, \(H_2O\)
Answer/Explanation
Ans: A
Question
Which row describes how an ionic bond forms between a sodium atom and a chlorine atom?
Answer/Explanation
Ans: D
Question
Which diagram shows the structure of an alloy?
Answer/Explanation
Ans: D
Question
Methane burns in oxygen to produce carbon dioxide and water.
What is the balanced equation for this reaction?
A \(CH_4 + 2O_2 \rightarrow 2CO_2 + 2H_2O\)
B \(CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O\)
C \(CH_4 + 2O_2 \rightarrow CO_2 + H_2O\)
D \(CH_4 + O_2 \rightarrow CO_2 + 2H_2O\)
Answer/Explanation
Ans: B
Question
What is the relative formula mass of magnesium nitrate, \(Mg(NO_3)_2\)?
A 74 B 86 C 134 D 148
Answer/Explanation
Ans: D
Question
In separate experiments, electricity was passed through concentrated aqueous sodium chloride
and molten lead(II) bromide.
What would happen in both experiments?
A A halogen would be formed at the anode.
B A metal would be formed at the cathode.
C Hydrogen would be formed at the anode.
D Hydrogen would be formed at the cathode.
Answer/Explanation
Ans: A
Question
Steel core aluminium cables are used for overhead electricity cables.
Which statement explains why these cables are used?
A Aluminium conducts electricity only when it surrounds a steel core.
B Aluminium conducts electricity and the steel core makes the cable stronger.
C Steel conducts electricity and is surrounded by aluminium because aluminium is an insulator.
D Steel conducts electricity and is surrounded by aluminium to stop the steel from corroding.
Answer/Explanation
Ans: B
Question
The complete combustion of propane is exothermic.
The equation for this reaction is shown.
\(C_3H_8 + 5O_2 \rightarrow 3CO_2 + 4H_2O\)
Which energy level diagram represents the complete combustion of propane?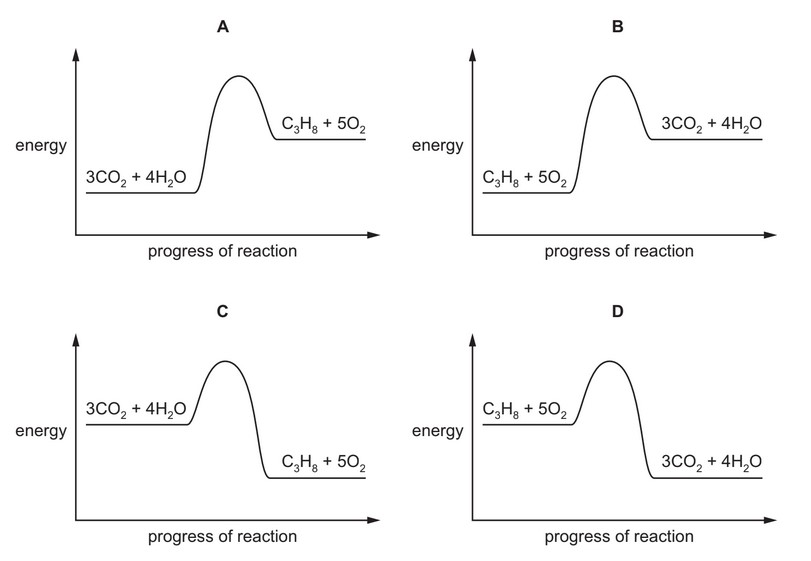
Answer/Explanation
Ans: D
Question
Which changes occur when hydrogen is burned in oxygen?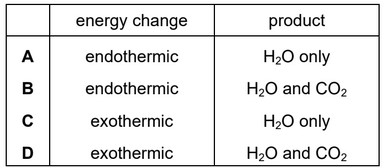
Answer/Explanation
Ans: C
Question
When sulfur is heated it undergoes a ……1…… change as it melts.
Further heating causes the sulfur to undergo a ……2…… change and form sulfur dioxide.
Which words complete gaps 1 and 2?
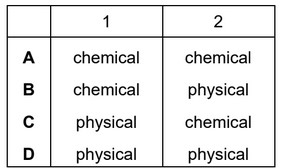
Answer/Explanation
Ans: C
Question
Zinc reacts with an acid to form a gas. The volume of gas produced is measured at intervals. The results are shown as curve Z.
The reaction is repeated in the presence of a catalyst.
Which curve shows the results for the catalysed reaction?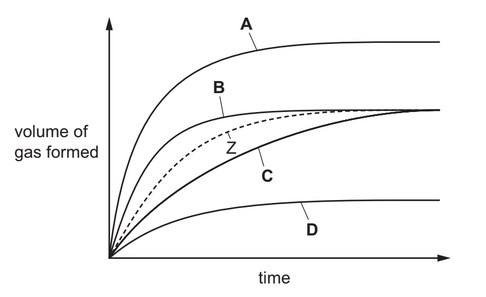
Answer/Explanation
Ans: B
Question
Which statement is correct?
A When anhydrous copper(II) sulfate is heated its colour changes to a deeper blue.
B When hydrated copper(II) sulfate is heated its colour changes to a deeper blue.
C When water is added to blue cobalt(II) chloride paper it turns pink.
D When water is added to pink cobalt(II) chloride paper it turns blue.
Answer/Explanation
Ans: C
Question
Three separate experiments are carried out on an aqueous solution of S.
The results are shown.
1 Magnesium does not react with the solution.
2 A gas is given off when ammonium sulfate is heated with the solution.
3 Methyl orange turns yellow when added to the solution.
What is S?
A hydrochloric acid
B sodium hydroxide
C sodium chloride
D sulfur dioxide
Answer/Explanation
Ans: B
Question
Element X forms an oxide, XO, that neutralises sulfuric acid.
Which row describes X and XO?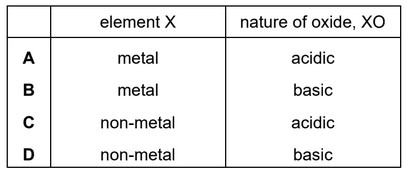
Answer/Explanation
Ans: B
Question
Copper(II) sulfate is prepared by adding excess copper(II) oxide to warm dilute sulfuric acid.
Which purification methods are used to obtain pure solid copper(II) sulfate from the reaction
mixture?
1 crystallisation
2 filtration
3 chromatography
4 distillation
A 1 and 4 B 1 and 2 C 2 and 3 D 3 and 4
Answer/Explanation
Ans: B
Question
Some reactions of element M are shown.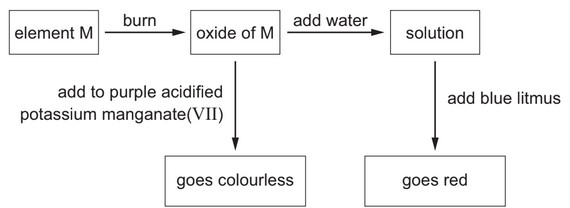
What is element M?
A carbon
B iron
C magnesium
D sulfur
Answer/Explanation
Ans: D
Question
Element X is in Group II of the Periodic Table.
Which statements about X are correct?
1 X is a metal.
2 X has two electrons in its outer shell.
3 X is a liquid at room temperature.
A 1 and 2 only B 1 and 3 only C 2 and 3 only D 1, 2 and 3
Answer/Explanation
Ans: A
Question
Why is helium used to fill balloons?
A Helium is monoatomic.
B Helium is in Group VIII of the Periodic Table.
C Helium has a full outer electron shell.
D Helium is less dense than air.
Answer/Explanation
Ans: D
Question
Which row describes the trend in properties of the elements in Group I as the group is descended?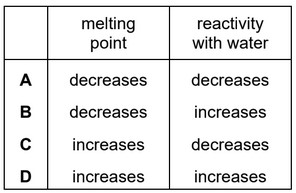
Answer/Explanation
Ans: B
Question
An element melts at \(1455^o\)C, has a density of 8.90 g / \(cm^3\) and forms a green chloride.
Where in the Periodic Table is this element found?
Answer/Explanation
Ans: C
Question
Some properties of metal J are listed.
● J does not react with cold water.
● J reacts with dilute hydrochloric acid.
● No reaction occurs when the oxide of J is heated with carbon.
What is J?
A copper
B iron
C magnesium
D sodium
Answer/Explanation
Ans: C
Question
Iron from a blast furnace is treated with oxygen and with calcium oxide to make steel.
Which substances in the iron are removed?
Answer/Explanation
Ans: A
Question
Which row describes a use of the metal and explains why it is used?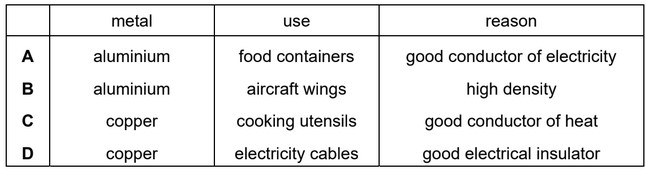
Answer/Explanation
Ans: C
Question
Ammonium chloride is heated with aqueous sodium hydroxide.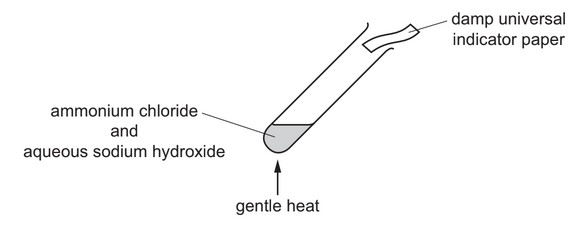
A gas is produced which turns damp universal indicator paper blue.
Which gas has been produced?
A ammonia
B hydrogen
C oxygen
D sulfur dioxide
Answer/Explanation
Ans: A
Question
Which two gases make up approximately 99% of clean, dry air?
A carbon dioxide and nitrogen
B carbon dioxide and oxygen
C nitrogen and oxygen
D argon and nitrogen
Answer/Explanation
Ans: C
Question
A student writes three statements about potassium nitrate, KNO3.
1 The relative formula mass of KNO3 is 101.
2 Potassium nitrate contains the three essential elements for plant growth.
3 Potassium nitrate could be used as a fertiliser.
Which statements are correct?
A 1 and 2 only B 1 and 3 only C 2 and 3 only D 1, 2 and 3
Answer/Explanation
Ans: B
Question
Which row describes the uses of sulfur and sulfur dioxide?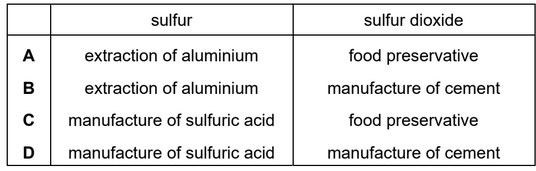
Answer/Explanation
Ans: C
Question
A white solid Z reacts with dilute hydrochloric acid to produce a gas.
The same gas is produced when compound Z is heated strongly.
What is Z?
A calcium
B calcium carbonate
C calcium hydroxide
D calcium oxide
Answer/Explanation
Ans: B
Question
Some information about compound L is listed.
1 L is an organic compound which contains four hydrogen atoms.
2 L is soluble in water.
3 An aqueous solution of L reacts with copper(II) carbonate to produce a gas.
What is L?
A methane
B ethene
C ethanoic acid
D ethanol
Answer/Explanation
Ans: C
Question
The structure of an organic molecule is shown.
Answer/Explanation
Ans: D
Question
Which compounds belong to the same homologous series?
A ethane and propane
B ethanoic acid and ethanol
C methane and ethene
D propene and ethanoic acid
Answer/Explanation
Ans: A
Question
Which statement about alkanes is correct?
A They burn in oxygen.
B They contain carbon, hydrogen and oxygen atoms.
C They contain double bonds.
D They contain ionic bonds.
Answer/Explanation
Ans: A
Question
Which structure represents poly(ethene)?
Answer/Explanation
Ans: D
Question
P, Q, R and S are four organic compounds.
P is an unsaturated hydrocarbon.
Q burns but otherwise is unreactive.
R contains a C–C single bond and a C=C double bond.
S undergoes addition polymerisation.
Which compounds are alkenes?
A P and R only
B P, R and S
C P, Q and S
D Q, R and S
▶️Answer/Explanation
Ans: B
Question
Which statement about petroleum fractions is correct?
A All petroleum fractions are used as fuels.
B Gas oil is used to make bottled gas for heating.
C Hydrocarbons in diesel have higher boiling points than hydrocarbons in gasoline.
D Molecules in kerosene are larger than molecules in fuel oil.
Answer/Explanation
Ans: C
Question
Which substance is a natural polymer?
A ethene
B Terylene
C nylon
D protein
Answer/Explanation
Ans: D
