Question
Give the name of the process that is used:
(a) to produce ammonia from nitrogen
(b) to separate nitrogen from liquid air
(c) to produce bromine from molten lead(II) bromide
(d) to separate an undissolved solid from an aqueous solution
(e) to produce amino acids from proteins
(f) to separate a mixture of amino acids.
Answer/Explanation
Answer:
(a) Haber (process)
(b) fractional distillation
(c) electrolysis
(d) filtration
(e) hydrolysis
(f) chromatography
Question
Complete the table to:
● deduce the number of protons, electrons and neutrons in the magnesium atom and copper ion
shown
● identify the atom or ion represented by the final row.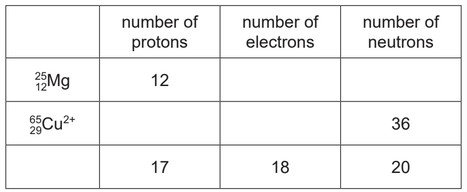
Answer/Explanation
Answer:
Mg: 12 and 13 (1)
\(Cu^{2+}: 29\) and 27 (1)
37(above)
and17(below) (1)
Cl (1)
1– (1)
Question
Potassium reacts with chlorine to form potassium chloride, KCl.
(a) Write a chemical equation for this reaction.
(b) Potassium chloride is an ionic compound.
Complete the diagram to show the electron arrangement in the outer shells of the ions present
in potassium chloride.
Give the charges on both ions.
(c) Molten potassium chloride undergoes electrolysis.
(i) State what is meant by the term electrolysis.
(ii) Name the products formed at the positive electrode (anode) and negative electrode
(cathode) when molten potassium chloride undergoes electrolysis.
anode ………………………………………………………………………………………………………………….
cathode ……………………………………………………………………………………………………………….
(d) Concentrated aqueous potassium chloride undergoes electrolysis.
(i) Write an ionic half-equation for the reaction at the negative electrode (cathode).
(ii) Name the product formed at the positive electrode (anode).
(iii) Name the potassium compound that remains in the solution after electrolysis.
(e) Complete the dot-and-cross diagram to show the electron arrangement in a molecule of
chlorine, \(Cl_2\).
Show the outer electrons only.
(f) The melting points and boiling points of chlorine and potassium chloride are shown.
(i) Deduce the physical state of chlorine at –75°C. Use the data in the table to explain your
answer.
physical state ……………………………………………………………………………………………………….
explanation ………………………………………………………………………………………………………….
(ii) Explain, in terms of structure and bonding, why potassium chloride has a much higher melting point than chlorine.
Your answer should refer to the:
● types of particle held together by the forces of attraction
● types of forces of attraction between particles
● relative strength of the forces of attraction.
Answer/Explanation
Answer:
(a) \(2K + Cl_2 → 2KCl\)
\(Cl_2\) on left hand side (1)
equation fully correct (1)
(b) K outer shell with 8 crosses (1)
Cl outer shell with 7 dots and 1 cross (1)
\(^+\) and – (1)
(c) (i) breakdown by (the passage of) electricity (1)
of an ionic compound in molten or aqueous (state) (1)
(ii) (anode) chlorine
(cathode)potassium
(d) (i) \(2H^+ + 2e(^–) → H_2\)
\(H^+\) and \(e(^–)\) on left hand side (1)
equation fully correct (1)
(ii) chlorine
(iii) potassium hydroxide (1)
(e) one shared pair of electrons and 6 non-bonding electrons on each chlorine atom
(f) (i) liquid (1)
BOTH melting point is below –75 oC AND boiling point is above –75 oC
OR
BOTH –75 oC is higher than –101 oC / melting point AND lower than –35 oC / boiling point
OR
–75 oC is between melting point or –101 oC and boiling point or –35 oC
(ii) ionic bonds in KCl (1)
attraction between molecules in \(Cl_2\) (1)
weaker attraction (between particles) in \(Cl_2\) ORA (1)
Question
Dinitrogen tetroxide, \(N_2O_4\), decomposes into nitrogen dioxide, \(NO_2\). The reaction is reversible.
A gas syringe containing a mixture of dinitrogen tetroxide and nitrogen dioxide gases was sealed
and heated. After reaching equilibrium the mixture was a pale brown colour.
(a) State what is meant by the term equilibrium.
(b) The plunger of the gas syringe is pushed in. The temperature does not change. The mixture
initially turns darker brown. After a few seconds the mixture turns lighter brown because the
equilibrium shifts to the left.
(i) Explain why the mixture initially turns darker brown.
(ii) Explain why the position of equilibrium shifts to the left.
(c) The forward reaction is endothermic.
(i) State what happens to the position of equilibrium when the temperature of the mixture is
increased.
(ii) State what happens to the rate of the forward reaction and the rate of the backward
reaction when the temperature of the mixture is increased.
rate of the forward reaction …………………………………………………………………………………….
rate of the backward reaction …………………………………………………………………………………
Answer/Explanation
Answer:
(a) the rate of forward reaction equals the rate of the reverse reaction (1)
concentrations of reactants and products are constant (1)
(b) (i) (increased pressure) nitrogen dioxide particles or molecules (forced) closer together
OR
same number of nitrogen dioxide particles or molecules in a smaller volume
(ii) fewer number of gas moles or molecules on left hand side or reactant side (of the equation) ORA
(c) (i) shifts to the right
(ii) increase / faster (1)
increase / faster (1)
Question
This question is about salts.
(a) Salts that are insoluble in water are made by precipitation.
● Lead(II) iodide, \(PbI_2\), is insoluble in water.
● All nitrates are soluble in water.
● All sodium salts are soluble in water.
You are provided with solid lead(II) nitrate, \(Pb(NO_3)_2\), and solid sodium iodide, NaI.
Describe how you would make a pure sample of lead(II) iodide by precipitation.
Your answer should include:
● practical details
● a chemical equation for the precipitation reaction.
(b) Nitrates decompose when heated.
(i) When hydrated zinc nitrate is heated, oxygen gas is given off.
Describe a test for oxygen.
test ……………………………………………………………………………………………………………………..
observations ………………………………………………………………………………………………………..
(ii) Complete the equation for the decomposition of hydrated zinc nitrate.
\(2Zn(NO_3)_2•6H_2O → …..ZnO + …..NO_2 + O_2 + …..H_2O\)
(c) Some sulfates are hydrated.
When hydrated sodium sulfate crystals, Na2SO4•xH2O, are heated, they give off water.
\(Na_2SO_4•xH_2O(s) → Na_2SO_4(s) + xH_2O(g)\)
A student carries out an experiment to determine the value of x in \(Na_2SO_4•xH_2O\).
step 1 Hydrated sodium sulfate crystals are weighed.
step 2 The hydrated sodium sulfate crystals are then heated.
step 3 The remaining solid is weighed.
(i) Describe how the student can check that all the water has been given off.
(ii) In an experiment, 1.61g of \(Na_2SO_4•xH_2O\) is heated until all the water is given off. The mass of \(Na_2SO_4\) remaining is 0.71g.
\([M_r : Na_2SO_4,142; H_2O,18]\)
Determine the value of x using the following steps.
● Calculate the number of moles of \(Na_2SO_4\) remaining.
………………………… mol
● Calculate the mass of \(H_2O\) given off.
………………………… g
● Calculate the number of moles of \(H_2O\) given off.
………………………… mol
● Determine the value of x.
x = …………………………
Answer/Explanation
Answer:
(a) (add) water (to both salts) (1)
dissolve both salts / make solutions (1)
filter (lead(II) iodide)(1)
wash (residue of lead(II) iodide) with water AND dry e.g. with filter paper / description of washing and drying (1)
\(Pb(NO_3)_2 + 2 NaI → 2NaNO_3 + PbI_2\)
OR \(Pb^{2+} + 2I^– → PbI_2\) (1)
(b) (i) glowing splint (1)
relights / rekindles (1)
(ii) 2ZnO(s) and \(4NO_2\)(g) (1)
\(12H_2O\)(g) (1)
(c) (i) heat again and weigh again / repeat steps 2 and 3 (1)
until mass is constant (1)
(ii) 0.005 (1)
0.9 (1)
(0.9 ÷ 18 =) 0.05 (1)
(0.05 ÷ 0.005 =) 10 (1)
Question
This question is about iron.
(a) Iron is extracted from its main ore in a blast furnace.
(i) Name the main ore of iron used in the blast furnace.
(ii) Name the substance that enters the blast furnace at A.
(iii) Name the substance that leaves the blast furnace at B.
(iv) Give two reasons for using coke in the blast furnace.
1 …………………………………………………………………………………………………………………………
2 …………………………………………………………………………………………………………………………
(b) Another ore of iron is iron pyrites, \(FeS_2\). Iron pyrites contains the positive ion, \(Fe^{2+}\).
Deduce the formula of the negative ion in \(FeS_2\).
(c) Iron is a transition element.
A list of properties of iron is shown.
● Iron is a good conductor of electricity.
● Iron forms soluble salts.
● Iron forms coloured compounds.
● Iron has variable oxidation states.
● Iron acts as a catalyst.
● Iron forms a basic oxide.
(i) Give two properties from the list in which iron differs from Group I elements.
1 …………………………………………………………………………………………………………………………
2 …………………………………………………………………………………………………………………………
(ii) Give two properties from the list in which iron is similar to Group I elements.
1 …………………………………………………………………………………………………………………………
2 …………………………………………………………………………………………………………………………
(d) Steel consists mainly of iron.
Iron forms rust when it reacts with water and oxygen.
Magnesium blocks can be attached to the bottom of steel boats. The magnesium does not
completely cover the steel.
(i) Explain how the magnesium blocks prevent iron from rusting.
(ii) Explain why replacing the magnesium blocks with copper blocks will not prevent the
bottom of the boat from rusting.
Answer/Explanation
Answer:
(a) (i) hematite
(ii) air
(iii) slag / calcium silicate
(iv) any two from:
• (coke)
releases heat (when it reacts with oxygen or reacts in air)
OR (acts as a) fuel
OR increases temperature (in the furnace) / heats (the furnace)
OR source of energy
• (coke or carbon monoxide)
reduces iron oxide
OR is a reducing agent
OR converts iron oxide to iron /
removes oxygen from iron oxide
• (coke)
reacts with oxygen to form carbon monoxide
OR reacts with carbon dioxide to form carbon monoxide
(b) \(S_2^{2-}\) or S-
(c) (i) any two from:
• (iron forms) coloured compounds
• (iron has) variable oxidation states
• (iron acts as a) catalyst
(ii) any two from:
• (iron is) good conductor of electricity
• (iron) forms a basic oxide
• (iron salts are) soluble (in water)
(d) (i) magnesium is more reactive than iron / steel ORA (1)
iron is not oxidised
OR
iron does not lose electrons
OR
magnesium loses electrons more easily than or in preference (to iron) ORA
OR
magnesium is oxidised more easily or reacts with oxygen more easily or corrodes more easily or in preference (to iron)
ORA (1)
(ii) copper is less reactive than iron / copper is lower in the reactivity series than iron ORA
Question
Many organic compounds contain carbon, hydrogen and oxygen only.
(a) An organic compound V has the following composition by mass.
C, 48.65%; H, 8.11%; O, 43.24%
Calculate the empirical formula of compound V.
empirical formula = …………………………
(b) Compound W has the empirical formula CH4O and a relative molecular mass of 32.
Calculate the molecular formula of compound W.
molecular formula = …………………………
(c) Compounds X and Y have the same general formula.
X and Y are both carboxylic acids.
Compound X has the molecular formula \(C_2H_4O_2\).
Compound Y has the molecular formula \(C_4H_8O_2\).
(i) Deduce the general formula of compounds X and Y.
(ii) Draw the structure of compound Y. Show all of the atoms and all of the bonds.
Name compound Y.
name …………………………………………………………………………………………………………………..
(iii) Give the name used to describe a ‘family’ of similar compounds with the same general
formula, similar chemical properties and the same functional group.
(d) Propene is an unsaturated hydrocarbon. The formula of propene is shown.
\(CH_3CH=CH_2\)
(i) State the colour change observed when propene is added to aqueous bromine.
from ……………………………………………………. to ……………………………………………………
(ii) Propene can be produced by cracking long chain alkanes.
Pentadecane, \(C_{15}H_{32}\), is cracked to produce an alkane and propene in a 1:2 molar ratio.
Complete the chemical equation for this reaction.
\(C_{15}H_{32}\) → ………………………………… + …………………………………
(iii) Propene can be converted into poly(propene).
Name the type of polymerisation that occurs when propene is converted into poly(propene).
(iv) Complete the diagram to show a section of poly(propene).
Answer/Explanation
Answer:
(a) 48.65 / 12 8.11 / 1 43.24 / 16 (1)
OR evaluation
4.05 8.11 2.7(0)
divide all by smallest
OR 1.5 : 3 : 1
OR
6 : 3 : 2 (1)
\(C_3H_6O_2\) (1) ALLOW symbols in any order
(b) ( \(M_r\) of \(CH_4O\) = 32)
\(CH_4O\) (1)
(c) (i) \(C_nH_{2n}O_2\)
OR
\(C_nH_{2n+1} COOH\)
(ii) butanoic acid (1)
fully displayed carboxylic acid group (1)
correct structure of butanoic acid showing all atoms and bonds (1)
(iii) homologous series
(d) (i) brown to colourless
(ii) \(C_9H_{20}\) (1)
\(2C_3H_6\) (1)
(iii) addition
(iv) 
any one repeat unit (1)
both repeat units fully correct (1)
