Questions 1
The boiling point of sodium is 890°C. What happens to sodium atoms as the temperature of a sample of sodium changes from 950 °C to 900°C?
A The atoms move more quickly and bonds are formed.
B The atoms move more quickly and bonds are neither broken nor formed.
C The atoms move more slowly and bonds are formed.
D The atoms move more slowly and bonds are neither broken nor formed.
▶️Answer/Explanation
Ans D
Questions 2
Which row shows the conditions for the particles of a gas colliding most frequently?
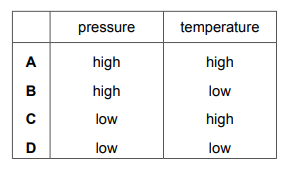
▶️Answer/Explanation
Ans A
Questions 3
The diagram shows some properties of particles in an atom. To which labelled part of the diagram do electrons belong?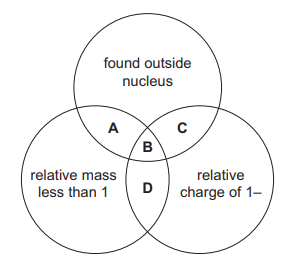
▶️Answer/Explanation
Ans B
Questions 4
Some properties of substances W, X, Y and Z are shown.
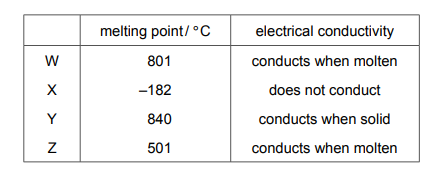
Which substances are ionic?
A W, X and Y
B W and Y only
C W and Z
D X and Z
▶️Answer/Explanation
Ans C
Questions 5
Atoms lose or gain electrons to become ions. Which row is correct?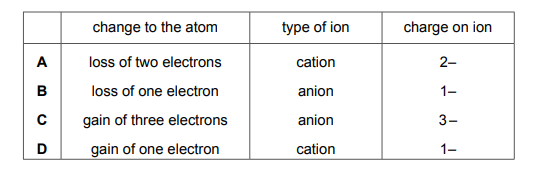
▶️Answer/Explanation
Ans C
Questions 6
A covalent molecule, M, contains four shared pairs of electrons. What is M?
A ammonia, \(NH_3\)
B hydrogen chloride, HCl
C methane, \(CH_4\)
D water, \(H_2O\)
▶️Answer/Explanation
Ans C
Questions 7
Which substance has a giant covalent structure?
A sodium chloride
B sodium
C ethane
D diamond
▶️Answer/Explanation
Ans D
Questions 8
Iron(III) oxide is reduced by carbon monoxide to produce iron and carbon dioxide. What is the balanced equation for this reaction?
A \(Fe_2O_3 + 2CO → 2Fe + 2CO_2\)
B \(Fe_2O_3 + 3CO → 2Fe + 3CO_2\)
C \(2Fe_2O_3 + 6CO → 2Fe + 6CO_2\)
D \(2Fe_2O_3 + 3CO → 4Fe + 3CO_2\)
▶️Answer/Explanation
Ans B
Questions 9
The equation for the reaction between magnesium and dilute hydrochloric acid is shown.
\(Mg + 2HCl → MgCl_2 + H_2\)
Which mass of magnesium chloride is formed when 48.0 g of magnesium completely reacts with excess dilute hydrochloric acid?
A 23.8 g
B 47.5 g
C 95.0 g
D 190 g
▶️Answer/Explanation
Ans D
Questions 10
Dilute sulfuric acid and lead(II) bromide are electrolysed separately. Which statements are correct?
1 Colourless gases are produced when dilute sulfuric acid is electrolysed.
2 Lead(II) bromide can be electrolysed when molten.
3 Lead is formed at the positive electrode when lead(II) bromide is electrolysed.
4 Sulfate ions are produced at the negative electrode when dilute sulfuric acid is electrolysed.
A 1 and 2
B 1 and 3
C 2 and 3
D 3 and 4
▶️Answer/Explanation
Ans A
Questions 11
Which statements about a hydrogen–oxygen fuel cell are correct?
1 The main form of energy released by the fuel cell is heat.
2 The reaction is a redox reaction.
3 An acidic gas is produced.
4 Water is the only chemical product.
A 1 and 3
B 1 and 4
C 2 and 3
D 2 and 4
▶️Answer/Explanation
Ans D
Questions 12
Which row describes what happens during an endothermic reaction?
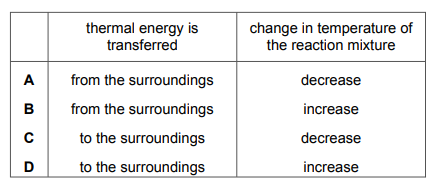
▶️Answer/Explanation
Ans A
Questions 13
Which diagram represents a chemical change?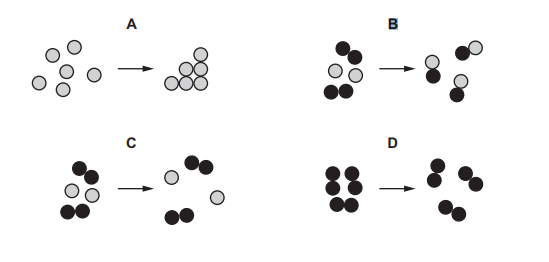
▶️Answer/Explanation
Ans B
Questions 14
A method used to investigate the rate of reaction of calcium carbonate with dilute hydrochloric acid under different conditions is shown. Place 50 \(cm^3\)
• of dilute hydrochloric acid in a conical flask.
• Add a known volume of water to the conical flask.
• Heat the conical flask to the required temperature.
• Add 1.0 g of calcium carbonate to the conical flask.
• Measure the time taken for the reaction to finish.
Which volume of water and which temperature give the shortest time taken for the reaction to finish?

▶️Answer/Explanation
Ans B
Questions 15
The rate of reaction between magnesium and hydrochloric acid is investigated. The total volume of hydrogen given off is measured at different times. A graph of the results is shown.
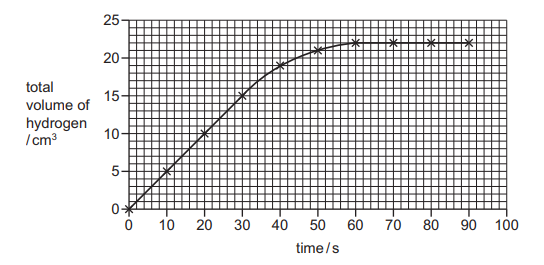
Which conclusions are correct?
1 The rate is fastest between 0 and 30 seconds.
2 The maximum volume of hydrogen given off is 22 \(cm^3\)
3 At 40 seconds, 20 \(cm^3\) of hydrogen is given off.
A 1 and 2 only
B 1 and 3 only
C 2 and 3 only
D 1, 2 and 3
▶️Answer/Explanation
Ans A
Questions 16
Water is added to anhydrous copper(II) sulfate. Which row describes the direction of energy change and the colour change of the mixture during the reaction?

▶️Answer/Explanation
Ans D
Questions 17
Which equation represents an oxidation reaction?
A \(CaCO_3 → CaO + CO_2\)
B \(4FeO + O_2 → 2Fe_2O_3\)
C \(2NO_2 → N_2O_4\)
D \(2P_2O_5 → P_4O_{10}\)
▶️Answer/Explanation
Ans B
Questions 18
A farmer treats a field with calcium hydroxide to make it less acidic. When the farmer adds ammonium nitrate fertiliser to the field immediately after the calcium hydroxide, the two substances react. Why does this reaction make the fertiliser less effective?
A It makes ammonia gas, so less nitrogen is absorbed by the soil.
B It makes an acid, making the soil acidic again.
C It makes nitrogen gas, so less nitrogen is absorbed by the soil.
D It makes the fertiliser too strong, stopping the plants growing well.
▶️Answer/Explanation
Ans A
Questions 19
Which statement about sodium oxide or nitrogen dioxide is correct?
A Nitrogen dioxide is a solid at room temperature.
B Nitrogen dioxide is acidic.
C Sodium oxide has a lower melting point than nitrogen dioxide.
D Sodium oxide is covalently bonded
▶️Answer/Explanation
Ans B
Questions 20
A titration method is used to prepare a pure soluble sulfate salt from dilute sulfuric acid. What is the other reagent?
A copper(II) oxide
B magnesium
C sodium hydroxide
D zinc carbonate
▶️Answer/Explanation
Ans C
Questions 21
Which row about elements in the Periodic Table is correct?
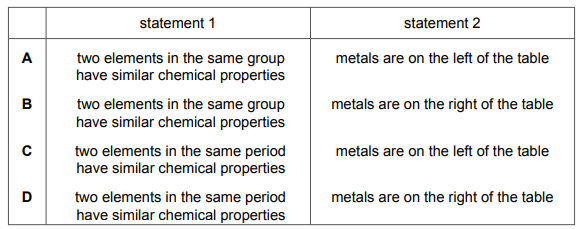
▶️Answer/Explanation
Ans A
Questions 22
The table gives some information about three elements in Group I of the Periodic Table.

Which row identifies the melting point and the density of rubidium? 
▶️Answer/Explanation
Ans B
Questions 23
Which statement describes a transition element?
A It is a dull grey metal that only forms white compounds.
B It is a high-density metal with a high melting point that is used as a catalyst.
C It is a low-density metal with a high melting point that reacts with steam to make hydrogen.
D It is a soft, shiny silver metal that reacts vigorously with water.
▶️Answer/Explanation
Ans B
Questions 24
The electronic configurations of helium, neon and argon are shown.
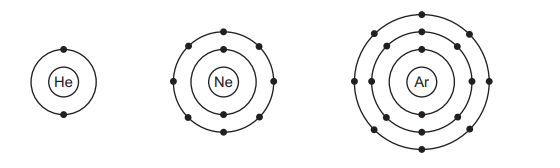
Which row describes these gases? 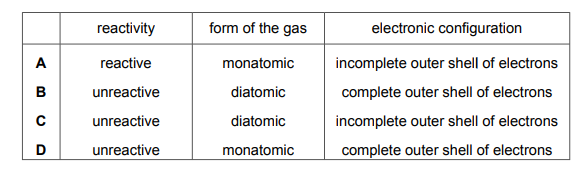
▶️Answer/Explanation
Ans D
Questions 25
X is a shiny silver-coloured solid at room temperature and pressure. X is a good conductor of heat and electricity when solid. Which statement about X is correct?
A X is an ionic compound or a metallic element.
B X is a metallic element or a non-metallic element.
C X is an alloy or a metallic element.
D X is an alloy or a non-metallic element.
▶️Answer/Explanation
Ans C
Questions 26
Which elements can be combined to produce an alloy?
1 magnesium and aluminium
2 nitrogen and oxygen
3 iron and carbon
4 copper and zinc
A 1, 3 and 4
B 1 and 2
C 2 and 3
D 4 only
▶️Answer/Explanation
Ans A
Questions 27
Three metals, L, M and N, are added separately to dilute hydrochloric acid and cold water. The results are shown.
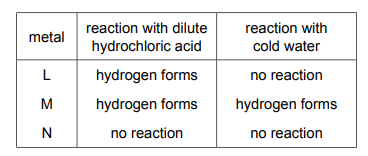
What is the order of reactivity of the metals? 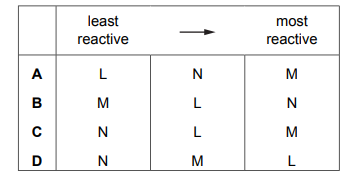
▶️Answer/Explanation
Ans C
Questions 28
Which reaction produces carbon dioxide?
A cracking of large hydrocarbon molecules
B photosynthesis
C reaction of a base with a carbonate
D thermal decomposition of calcium carbonate
▶️Answer/Explanation
Ans D
Questions 29
A sample of air containing four gases only is analysed. 99.0% of the sample contains the two main gases in the same percentages as in clean, dry air. The remaining 1.0% of the sample contains argon and carbon dioxide. The gas that makes up 0.1% of the sample turns limewater milky. Which row shows the percentage composition of the sample of air?
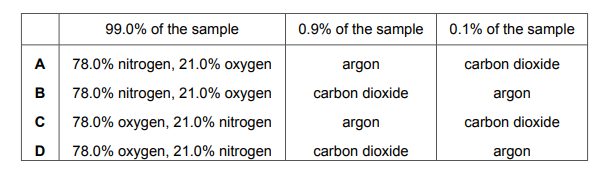
▶️Answer/Explanation
Ans A
Questions 30
Which substance contains two elements that are found in NPK fertilisers?
A ammonium chloride
B calcium hydroxide
C potassium nitrate
D sodium phosphate
▶️Answer/Explanation
Ans C
Questions 31
Which statement about sulfur is correct?
A When sulfur is burned, it produces a substance that causes acid rain.
B Sulfur is produced by the thermal decomposition of limestone.
C Compounds of sulfur make up approximately 1% of unpolluted air.
D Sulfur is a member of the family of elements called halogens.
▶️Answer/Explanation
Ans A
Questions 32
What are two adverse effects of particulates in the air?
1 acid rain
2 cancer
3 photochemical smog
4 respiratory problems
A 1 and 3
B 1 and 4
C 2 and 3
D 2 and 4
▶️Answer/Explanation
Ans D
Questions 33
Which formula represents a compound that is a member of the homologous series of alkanes?
A \(C_2H_4\)
B \(C_3H_6\)
C \(C_4H_8\)
D \(C_5H_{12}\)
▶️Answer/Explanation
Ans D
Questions 34
Which statement about ethane is correct?
A It rapidly decolourises aqueous bromine.
B It does not burn.
C It forms long-chain compounds called polymers.
D It only contains single bonds between its atoms.
▶️Answer/Explanation
Ans D
Questions 35
Which raw material is used to make ethanol by fermentation?
A carbon dioxide
B ethene
C glucose
D natural gas
▶️Answer/Explanation
Ans C
Questions 36
Which statement about ethanoic acid is correct?
A It contains a –COOH group.
B It has a pH greater than pH 7.
C It reacts with sodium carbonate to form hydrogen gas.
D It reacts with copper to form copper(II) ethanoate.
▶️Answer/Explanation
Ans A
Questions 37
Which statement explains why the disposal of plastic waste leads to environmental problems?
A Plastic waste forms toxic gases when it is burned.
B Plastic waste contains many small molecules.
C Plastic waste rapidly dissolves in the oceans.
D Plastic waste reacts with both acids and bases.
▶️Answer/Explanation
Ans A
Questions 38
Substance X and four known substances, P, Q, R and S, are analysed by chromatography. The chromatogram produced is shown.

Which statement about X is correct?
A It is a mixture of P, Q and S.
B It contains P and S only.
C It contains P, S and another unknown substance.
D It is a mixture of Q, R and S.
▶️Answer/Explanation
Ans C
Questions 39
Copper is insoluble in water. Copper(II) oxide is a solid that is insoluble in water but reacts with dilute hydrochloric acid. Which method is used to separate copper from a mixture of copper and copper(II) oxide?
A dissolve the mixture in water then filter
B dissolve the mixture in water then crystallise
C react the mixture with dilute hydrochloric acid then filter
D react the mixture with dilute hydrochloric acid then crystallise
▶️Answer/Explanation
Ans C
Questions 40
A salt, S, is dissolved in water and three tests are carried out on the solution formed.

What is the identity of S?
A copper(II) chloride
B copper(II) sulfate
C iron(II) chloride
D iron(II) sulfate
▶️Answer/Explanation
Ans D
