Question
(a) The diagram shows part of the Periodic Table.
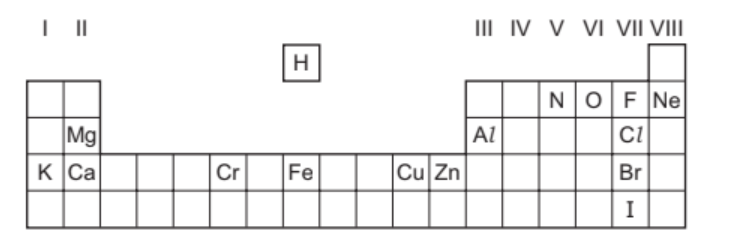
Answer the following questions using only the symbols of the elements in the diagram. Each symbol may be used once, more than once or not at all.
State the symbol of the element that:
(i) is a monoatomic gas at room temperature[1]
(ii) is a liquid at room temperature[1]
(iii) forms a stable ion of type $X^{2-}$[1]
(iv) is extracted from hematite[1]
(v) forms an ion whose aqueous solution gives a grey-green precipitate on addition of aqueous ammonia.[1]
(b) Magnesium has several naturally occurring isotopes.
(i) State the meaning of the term isotopes.[2]
(ii) An isotope of magnesium is shown.
${ }_{12}^{26} \mathrm{Mg}$
Deduce the number of protons and neutrons in this isotope.
number of protons………………………………………………..
number of neutrons………………………………………….[2]
(c) Complete the electronic structure of a magnesium atom.
(c) Complete the electronic structure of a magnesium atom.
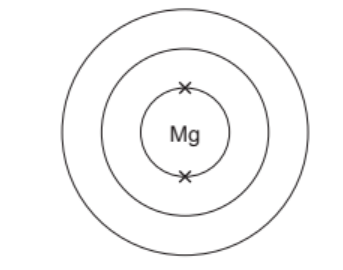 [1] [Total: 10]
[1] [Total: 10]
▶️Answer/Explanation
Ans:
(a)(i) Ne / neon
(a)(ii) $\mathrm{Br} /$ Bromine $/ \mathrm{Br}_2$
(a)(iii) O / oxygen
(a)(iv) Fe / iron
(a)(v) $\mathrm{Cr} /$ chromium $/ \mathrm{Cr}^{3 *}$
(b)(i) atoms (1)
(with the) same number of protons but different numbers of neutrons / (with the) same atomic number but different mass
number / (with the) same atomic number but different nucleon number (1)
(b)(ii) protons = 12 (1)
neutrons = 14 (1)
(c) 8 electrons in second shell and 2 electrons in outer shell
Question
The table shows the mass of air pollutants, in nanograms, in $1000 \mathrm{~cm}^3$ samples of air taken over a four month period.
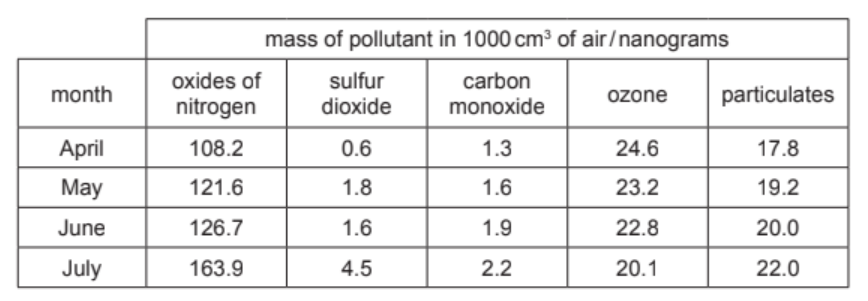
(a) Answer these questions using only the information in the table.
(i) Name the pollutant that shows a decrease in concentration between April and July.[1]
(ii) Name the pollutant present in the lowest concentration in May.[1]
(iii) Calculate the mass of sulfur dioxide in $250 \mathrm{~cm}^3$ of the sample of air taken in April.
nanograms [1]
(b) Oxides of nitrogen are produced when oxygen combines with nitrogen during thunderstorms.
(i) State one other source of oxides of nitrogen in the air.[1]
(ii) Give one adverse effect of oxides of nitrogen on health.[1]
(iii) Complete the chemical equation for the reaction of nitrogen with oxygen to form nitrogen dioxide.
$
+2 \mathrm{O}_2 \rightarrow \ldots . . \mathrm{NO}_2
$
(c) Particulates are tiny solid particles in the air.
The movement of these particles is shown by the arrows in the diagram.
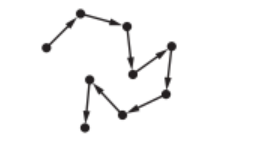 State the name given to this random motion of particles.
State the name given to this random motion of particles.
……………………………………………………………………………………………………………………………. [1] [Total: 8]
▶️Answer/Explanation
Ans:
(a)(i) ozone
a)(ii) carbon monoxide / CO
(a)(iii) 0.15 (ng)
(b)(i) car exhausts / car engines / high temperature furnaces
(b)(ii) irritation of lungs / irritates nose / irritates throat / irritates eyes / asthma
(b)(iii) $\begin{aligned} & \mathrm{N}_2 \text { (on left) } \\ & 2\left(\mathrm{NO}_2\right) \text { (on right) }\end{aligned}$
(c) Brownian (motion)
Question
Some properties of four substances, A, B, C and D, are shown in the table.
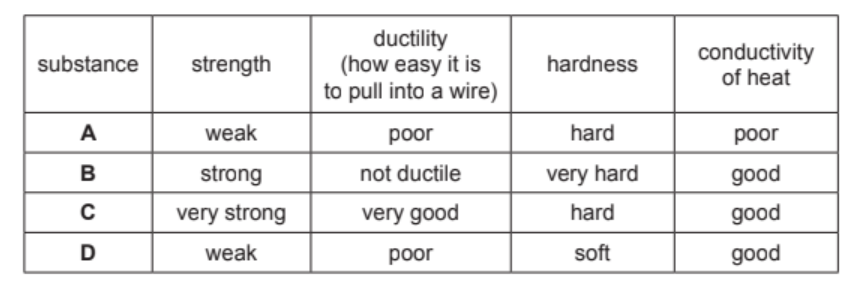
Answer these questions using only the information in the table.
(a) State which substance, A, B, C or D, is best used in the core of an overhead electricity cable.
Explain your answer.
substance ………………………………………………………………………………………………………………….
explanation ………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………………….
[3]
(b) State which substance, A, B, C or D, is best used for the tip of a drill.
Explain your answer.
substance ………………………………………………………………………………………………………………….
explanation ………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………. [3] [Total: 6]
▶️Answer/Explanation
Ans:
(a) C (1)
very strong (1)
very good ductility / best ductility (1)
(b) B (1)
very hard (1)
strong / good conductor of heat (1)
Question
4 The structure of tartaric acid is shown.
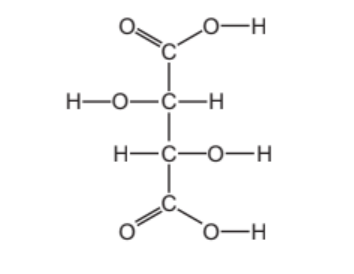
(a) (i) On the structure, draw a circle around one alcohol functional group. [1]
(ii) Deduce the formula of tartaric acid to show the number of carbon, hydrogen and oxygen atoms.
……………………………………………………………………………………………………………………… [1]
(iii) Complete the table to calculate the relative molecular mass of tartaric acid. Use your Periodic Table to help you.
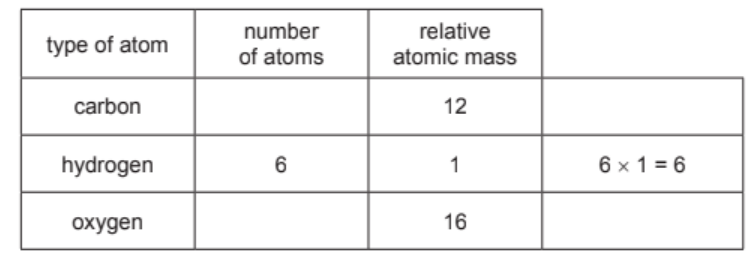
relative molecular mass = ………………………… [2]
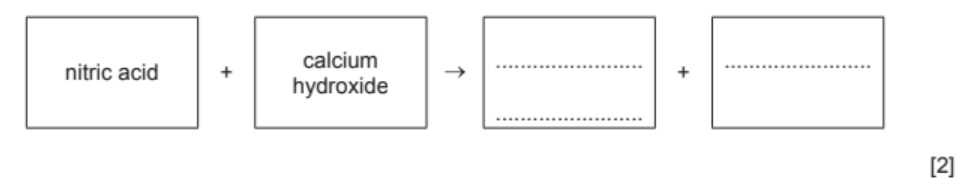
(b) Acids react with bases such as calcium hydroxide.
(i) Complete the word equation for the reaction of nitric acid with calcium hydroxide.
(ii) An aqueous solution of calcium hydroxide is alkaline.
Identify which one of these $\mathrm{pH}$ values represents the $\mathrm{pH}$ of an alkaline solution.
Draw a circle around the correct answer.
pH 1 pH 4 pH 7 pH 10 [1]
(iii) One way of determining $\mathrm{pH}$ is to use a $\mathrm{pH}$ meter.
Describe one other way of determining $\mathrm{pH}$.[2]
(iv) Farmers spread calcium hydroxide (slaked lime) on fields where crops are grown. Explain why.[1]
(c) Calcium carbonate undergoes thermal decomposition.
$
\mathrm{CaCO}_3 \rightarrow \mathrm{CaO}+\mathrm{CO}_2
$
(i) State the meaning of the term thermal decomposition.[2]
(ii) Complete the energy level diagram for the thermal decomposition of calcium carbonate by writing these words on the diagram:
- reactant
- products.
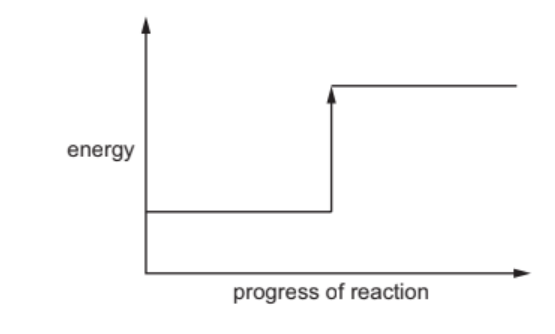
(iii) Explain, using information on the energy level diagram, how you know that this reaction is endothermic. [1] [Total: 14]
▶️Answer/Explanation
Ans:
4(a)(i) circle around one or both alcoholic O-H groups
4(a)(ii) $\mathrm{C}_4 \mathrm{H}_6 \mathrm{O}_6$
4(a)(iii) 150 (2)
If two marks not scored, 1 mark for 4 × 12 = 48 / 6 × 16 = 96
4(b)(i) calcium nitrate (1)
water (1)
4(b)(ii) pH 10 1
4(b)(iii) universal indicator (1)
compare colour with colour chart (1)
4(b)(iv) crops don’t grow if soil too acidic / plants don’t grow (well) in acidic conditions / plants need particular pH to grow best
4(c)(i) breakdown of a compound (1)
by heating / by high temperature (1)
4(c)(ii) reactants on left bottom line and products on top right line 1
4(c)(iii) the reactant has less energy than the products
Question
Ethene is an alkene.
(a) Draw the structure of ethene to show all of the atoms and all of the bonds.[1]
(b) Ethene reacts with aqueous bromine.
State the colour change observed when ethene reacts with aqueous bromine.
from…………………………..to……………………….[2]
(c) Ethene reacts with steam.
(i) Name and give the formula of the product of this reaction.
name…………………………..
formula…………………………………. [2]
(ii) Identify the type of chemical reaction that occurs when ethene reacts with steam.
Draw a circle around the correct answer.
addition fermentation polymerisation neutralisation [1]
(d) Alkenes are produced by cracking hydrocarbons.
Complete the chemical equation for the cracking of the hydrocarbon, $\mathrm{C}_{16} \mathrm{H}_{34}$, to produce an alkene and one other product.
$
\mathrm{C}_{16} \mathrm{H}_{34} \rightarrow \mathrm{C}_6 \mathrm{H}_{12}+
$
(e) Poly(ethene) is produced by combining many ethene molecules.
(i) Name the general term used to describe the small molecules which combine to form a polymer.[1]
(ii) Nylon is a polymer.
State one use for nylon.[1]
(iii) Describe one pollution problem caused by non-biodegradable plastics.[1] [Total: 10]
▶️Answer/Explanation
Ans:
(a) 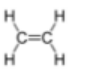
(b)orange (1)
to colourless (1)
(c)(i) ethanol (1)
$\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH} / \mathrm{C}_2 \mathrm{H}_6 \mathrm{O}(1)$
(c)(ii) addition
(d)$\mathrm{C}_{10} \mathrm{H}_{22}$
(e)(i) monomer(s)
(e)(ii) ropes / clothing / fishing lines / tennis racquets / tents / nets
(e)(iii) chokes animals / blocks digestive system in birds / animals trapped (inside bottles) / eyesore / fills landfill sites
Question
Electrolysis is used to extract metals from metal compounds.
(a) Describe the electrolysis of molten lead(II) bromide. In your answer include:
- a labelled diagram of the apparatus used
- the names of the products formed at the positive and the negative electrodes.
positive electrode…………………………..
negative electrode…………………………….. [5]
(b) Use the kinetic particle model to describe the arrangement and motion of the particles in molten (liquid) lead.
arrangement…………………………………………
motion……………………………………………[2]
(c) Lead is a metal which is soft and has a relatively low melting point.
State two other physical properties of metals such as lead.
1 ……………………………………………………………………………………………………………………………….
2 ………………………………………………………………………………………………………………………………. [2]
(d) Lead is formed when red lead oxide, $\mathrm{Pb}_3 \mathrm{O}_4$, is heated with carbon.
$
\mathrm{Pb}_3 \mathrm{O}_4+4 \mathrm{C} \rightarrow 3 \mathrm{~Pb}+4 \mathrm{CO}
$
Explain how this equation shows that $\mathrm{Pb}_3 \mathrm{O}_4$ has been reduced. [1] [Total: 10]
▶️Answer/Explanation
Ans:
(a) one mark each for:
• positive electrode: bromine / Br2
• negative electrode: lead / Pb
AND
one mark each for any three of:
• 2 electrodes dipping into liquid
• electrolyte labelled (as electrolyte or lead bromide)
• power pack or batteries labelled and leads connected correctly to electrodes
• electrodes labelled anode and cathode / electrodes labelled positive and negative electrodes
(b) arrangement: random / no arrangement (1)
motion: sliding over each other / random (1)
(c) one mark each for any two of:
• malleable
• ductile
• conducts electricity / conducts heat
• shiny (when freshly cut)
(d) $\mathrm{Pb}_3 \mathrm{O}_4$ loses oxygen/red lead loses oxygen/lead oxide loses oxygen
Question
Catalysts increase the rate of chemical reactions.
(a) Name the type of metals often used as catalysts. [1]
(b) A student investigated the reaction of zinc powder with excess dilute hydrochloric acid in the absence of a catalyst.
$
\mathrm{Zn}+2 \mathrm{HCl} \rightarrow \mathrm{ZnCl}_2+\mathrm{H}_2
$
The student measured the volume of hydrogen gas produced at 10 second intervals. The graph shows the results.

Answer these questions using information from the graph.
(i) Deduce the volume of hydrogen produced in the first 25 seconds of the experiment.
volume = ………………………… cm3[1]
(ii) Explain why no more hydrogen is produced after 50 seconds. ……………………………………………………………………………………………………………………… [1]
(iii) The experiment is repeated using a catalyst.
Draw a line on the grid to show how the volume of hydrogen changes with time when a catalyst is used.
All other conditions stay the same. [2]
(iv) Describe what effect the following changes have on the rate of the reaction.
- The concentration of hydrochloric acid is decreased.
All other conditions stay the same.
- Large pieces of zinc are used.
All other conditions stay the same. [5]
(c) (i) Describe a test for hydrogen.
test………………………….
result……………………………….. [3]
(ii) State one use of hydrogen. [1] [Total: 10]
▶️Answer/Explanation
Ans:
(a) transition (elements)
(b)(i) $70 \mathrm{~cm}^3$
(b)(ii) the reaction has finished / all the zinc has been used up 1
(b)(iii) Line starts at 0, 0 and steeper gradient (1)
Line levels out at 96 cm3 hydrogen and before the line already on the grid (1)
(b)(iv) concentration: (rate) decreases / gets slower (1)
particle size: (rate) decreases / gets slower (1)
(c)(i) lighted splint (1)
pops / explodes (1)
(c)(ii) fuel
Question
This question is about metals and compounds of metals.
(a) Identify two correct statements about transition elements. Tick two boxes.

(b) The table compares the ease of reduction of some metal oxides with carbon.

Put the four metals in order of their reactivity.
Put the least reactive metal first.

(c) Crystals of copper(II) sulfate, $\mathrm{CuSO}_4 \cdot 5 \mathrm{H}_2 \mathrm{O}$, can be prepared by heating excess copper(II) oxide powder with dilute sulfuric acid.
(i) Describe how to prepare a sample of pure dry copper(II) sulfate crystals after the reaction is complete.
In your answer describe how to:
- remove the excess copper(II) oxide from the reaction mixture
- crystallise the copper(II) sulfate
- dry the crystals. [4]
(ii) Identify the word that best describes copper(II) sulfate.
Draw a circle around the correct answer.
acid $\bigcirc$
halogen $\bigcirc$
polymer $\bigcirc$
salt $\bigcirc$ [1]
(d) A few drops of water are added to a sample of solid anhydrous copper(II) sulfate, $\mathrm{CuSO}_4$.
(i) The reaction is reversible.
$$
\mathrm{CuSO}_4+5 \mathrm{H}_2 \mathrm{O} \quad\square\quad \mathrm{CuSO}_4 \cdot 5 \mathrm{H}_2 \mathrm{O}
$$
Draw in the box, the sign for a reversible reaction. [1]
(ii) State the colour change observed when water is added to anhydrous copper(II) sulfate.
from —————– to ——————————– [2] [Total: 12]
▶️Answer/Explanation
Ans:
(a)$2^{\text {nd }}$ box down ticked (have high densities) (1)
$4^{\text {th }}$ box down ticked (high melting points) (1)
(b) copper<cobalt<manganese<magnesium (2)
If 2 marks not scored one mark for one consecutive pair reversed / all reversed
(c)(i) one mark each for any four of:
• filter (off copper oxide)
• warm (or heat or evaporate) filtrate to point of crystallisation / warm filtrate to form saturated solution / heat filtrate until
crystals start to form
• filter off crystals / pick out crystals
• wash crystals with cold water / wash crystals with (organic) solvent
• dry crystals with filter paper
(c)(ii) salt
(d)(i) ⇌
8(d)(ii) white (1)
(to) blue (1)
