Question
Decane has a freezing point of –30 °C and a boiling point of 174 °C.
A small sample of decane is placed in an open beaker in an oven at a temperature of 120 °C and
at atmospheric pressure for 24 hours.
What happens to the sample of decane?
A It boils.
B It evaporates.
C It melts.
D It sublimes.
Answer/Explanation
Ans: B
Question
A student put exactly 25.00 \(cm^3\) of dilute hydrochloric acid into a conical flask.
The student added 2.5 g of solid sodium carbonate and measured the change in temperature of
the mixture.
Which apparatus does the student need to use?
A balance, measuring cylinder, thermometer
B balance, pipette, stopwatch
C balance, pipette, thermometer
D burette, pipette, thermometer
Answer/Explanation
Ans: C
Question
A student separates sugar from pieces of broken glass by dissolving the sugar in water and filtering off the broken glass.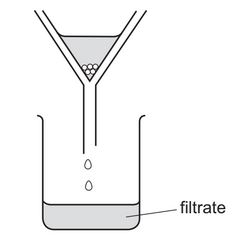
What is the filtrate?
A broken glass only
B broken glass and sugar solution
C pure water
D sugar solution
Answer/Explanation
Ans: D
Question
Two isotopes of carbon are \(^{12}C\) and \(^{14}C\).
Which statement about these two isotopes is correct?
A Their electronic structure is different.
B They have different numbers of nucleons.
C They have different numbers of protons.
D They have the same number of neutrons.
Answer/Explanation
Ans: B
Question
Which description of brass is correct?
A alloy
B compound
C element
D non-metal
Answer/Explanation
Ans: A
Question
The element livermorium, Lv, was discovered in the year 2000.
Which statement predicts what will happen to an Lv atom when it forms an \(Lv^{2–}\) ion?
A The atom will gain two electrons.
B The atom will lose two electrons.
C The atom will lose two protons.
D The atom will gain two protons.
Answer/Explanation
Ans: A
Question
Which substance is a diatomic covalent compound?
A \(Cl_2\) B HCl C \(H_2O\) D MgO
Answer/Explanation
Ans: B
Question
Which statement about carbon is correct?
A Diamond and graphite both have simple molecular structures.
B Diamond and graphite are both used to make cutting tools.
C Each carbon atom in diamond is bonded to three other carbon atoms.
D Graphite conducts electricity and has a giant covalent structure.
Answer/Explanation
Ans: D
Question
The formula of sodium chlorate(V) is \(NaClO_3\).
What is the relative formula mass of sodium chlorate(V), \(NaClO_3\)?
A 52.0 B 74.5 C 106.5 D 223.5
Answer/Explanation
Ans: C
Question
Which statements about the products of electrolysis, using inert electrodes, are correct?
1 When molten lead(II) bromide is electrolysed, bromine is formed at the cathode.
2 When dilute sulfuric acid is electrolysed, oxygen is formed at the anode.
3 When concentrated aqueous sodium chloride is electrolysed, sodium is formed at
the cathode.
4 When concentrated hydrochloric acid is electrolysed, chlorine is formed at the
anode.
A 1 and 2 B 1 and 3 C 2 and 4 D 3 and 4
Answer/Explanation
Ans: C
Question
The temperature decreases when aqueous ethanoic acid reacts with solid sodium carbonate to
form a salt.
Which type of reaction and energy change occur?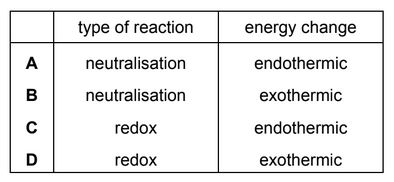
Answer/Explanation
Ans: A
Question
Which gas is used as a fuel?
A helium
B hydrogen
C nitrogen
D oxygen
Answer/Explanation
Ans: B
Question
Solid copper(II) carbonate reacts with dilute sulfuric acid.
\(CuCO_3 + H_2SO_4 → CuSO_4 + CO_2 + H_2O\)
The rate of the reaction can be changed by varying the conditions.
Which changes always increase the rate of this chemical reaction?
1 increasing the concentration of sulfuric acid
2 increasing the size of the pieces of copper(II) carbonate
3 increasing the temperature
4 increasing the volume of sulfuric acid
A 1, 3 and 4 B 1 and 3 only C 2 and 3 D 3 and 4 only
Answer/Explanation
Ans: B
Question
Some changes are shown in the table.
In which rows are the changes described correctly?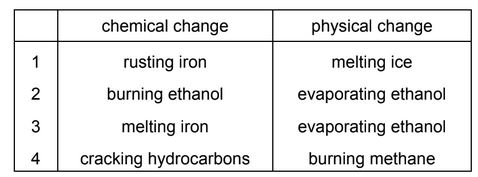
A 1 and 2 B 1 and 3 C 2 and 4 D 3 and 4
Answer/Explanation
Ans: A
Question
X is a pink solid.
Y is a blue solid.
When X is heated, water is produced and the solid turns blue.
When water is added to Y, the solid turns pink.
What are X and Y?
Answer/Explanation
Ans: B
Question
Carbon reacts with carbon dioxide as shown.
\(CO_2 + C → 2CO\)
Which statement about this reaction is correct?
A Carbon dioxide and carbon are both oxidised.
B Carbon dioxide and carbon are both reduced.
C Carbon dioxide is reduced and carbon is oxidised.
D Carbon dioxide is oxidised and carbon is reduced.
Answer/Explanation
Ans: C
Question
Which substances do not produce water as a product when they are reacted together?
A calcium hydroxide and ammonium chloride
B calcium carbonate and dilute hydrochloric acid
C copper(II) oxide and dilute nitric acid
D zinc and dilute sulfuric acid
Answer/Explanation
Ans: D
Question
The surface of magnesium ribbon reacts with the air to form magnesium oxide.
Which statement explains why the layer of magnesium oxide is removed by dilute hydrochloric
acid?
A Magnesium is a base.
B Magnesium ribbon reacts with hydrochloric acid.
C Magnesium oxide is a base.
D Magnesium oxide is an acid.
Answer/Explanation
Ans: C
Question
Copper(II) chloride crystals are made by adding solid copper(II) carbonate to dilute
hydrochloric acid until no more dissolves.
Which process is used to obtain pure copper(II) chloride crystals from the mixture?
A distillation of the mixture
B evaporation of the mixture
C filtration followed by drying of the residue
D filtration followed by evaporation of the filtrate
Answer/Explanation
Ans: D
Question
Which statement about aqueous sodium hydroxide is correct?
A When it is added to a solution containing sulfate ions, a white precipitate is formed.
B When it is added to a solution of copper(II) ions, a blue precipitate is formed which dissolves in excess to give deep blue solution.
C When it is added to a solution of iron(II) ions, a green precipitate is formed which does not dissolve in excess.
D When it is added to ammonium chloride, a gas is produced which turns blue litmus red.
Answer/Explanation
Ans: C
Question
A period of the Periodic Table is shown.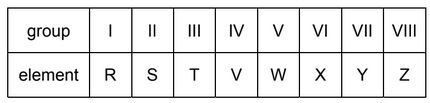
The letters are not their chemical symbols.
Which statement is correct?
A Element R does not conduct electricity.
B Elements R and Y react together to form an ionic compound.
C Element Z exists as a diatomic molecule.
D Element Z reacts with element T.
Answer/Explanation
Ans: B
Question
What are the products of the reaction between sodium and water?
A hydrogen and sodium hydroxide
B hydrogen and sodium oxide
C oxygen and sodium hydroxide
D oxygen and sodium oxide
Answer/Explanation
Ans: A
Question
Element X has a high density, a high melting point and a high electrical conductivity.
It forms many coloured compounds.
Element X and many of its compounds act as catalysts.
What could be the atomic number of X?
A 19 B 26 C 33 D 35
Answer/Explanation
Ans: B
Question
The noble gases are in Group VIII of the Periodic Table.
Which statement explains why noble gases are unreactive?
A They all have eight electrons in their outer shells.
B They all have full outer shells.
C They are all gases.
D They are all monoatomic.
Answer/Explanation
Ans: B
Question
Which statement is correct for all metals?
A They conduct electricity when molten.
B They gain electrons when they form ions.
C They have a low density.
D They have a low melting point.
Answer/Explanation
Ans: A
Question
Which statement about the extraction of metals is correct?
A Aluminium is extracted from the ore bauxite by electrolysis.
B Aluminium is extracted from the ore hematite by electrolysis.
C Iron is extracted from the ore bauxite by electrolysis.
D Iron is extracted from the ore hematite by electrolysis.
Answer/Explanation
Ans: A
Question
Which row identifies a use of mild steel and a use of stainless steel?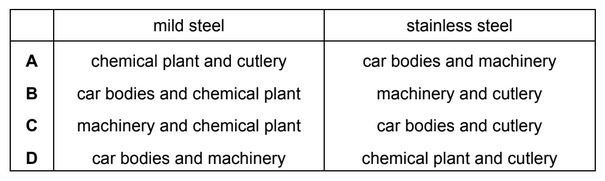
Answer/Explanation
Ans: D
Question
The diagrams show some uses of water in the home.
For which uses is it important for the water to have been treated?
A 1 only B 2 only C 3 only D 1, 2 and 3
Answer/Explanation
Ans: A
Question
Which air pollutants can cause damage to buildings made of limestone?
1 carbon monoxide
2 lead compounds
3 oxides of nitrogen
4 sulfur dioxide
A 1 and 2 B 1 and 4 C 2 and 3 D 3 and 4
Answer/Explanation
Ans: D
Question
Which statement about fertilisers is correct?
A Ammonium sulfate, \((NH_4)_2SO_4\), is a better fertiliser than ammonium nitrate, \(NH_4NO_3\), because it contains more oxygen.
B Ammonium phosphate, \((NH_4)_3PO_4\), is a good fertiliser because it contains hydrogen.
C Potassium nitrate, \(KNO_3\), is a good fertiliser because it provides potassium and nitrogen.
D Urea, \((NH_2)_2CO\), is a good fertiliser because it contains carbon.
Answer/Explanation
Ans: C
Question
Sulfur burns to make sulfur dioxide.
Which row describes a source of sulfur and a use of sulfur dioxide?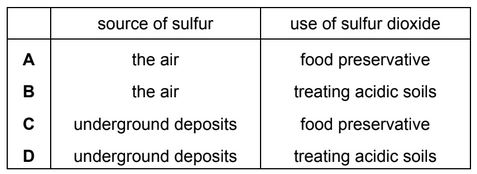
Answer/Explanation
Ans: C
Question
Lime (calcium oxide) is used to treat waste water from a factory.
Which substance is removed by the lime?
A ammonia
B sodium chloride
C sodium hydroxide
D sulfuric acid
Answer/Explanation
Ans: D
Question
Which compound is correctly named?
Answer/Explanation
Ans: B
Question
Fuel X produces carbon dioxide and water when it is burned in air. So does fuel Y.
What could X and Y be?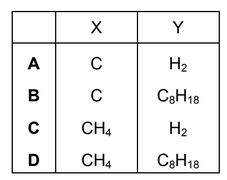
Answer/Explanation
Ans: D
Question
Which hydrocarbon is the main constituent of natural gas?
A butane
B ethane
C methane
D propane
Answer/Explanation
Ans: C
Question
Which statements about ethene are correct?
1 It contains a C=C bond.
2 It does not decolourise bromine water.
3 Its molecules can join together to form long chain compounds.
A 1, 2 and 3 B 1 and 2 only C 1 and 3 only D 2 and 3 only
Answer/Explanation
Ans: C
Question
Part of the structure of a very large molecule is shown.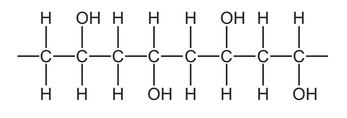
Which term describes the small unit used to make this molecule?
A hydrocarbon
B monomer
C polymer
D saturated
Answer/Explanation
Ans: B
Question
What is the total number of single covalent bonds in a molecule of ethanol?
A 5 B 6 C 7 D 8
Answer/Explanation
Ans: D
Question
Which statement about aqueous ethanoic acid is correct?
A It reacts with magnesium to produce a salt and hydrogen.
B It reacts with sodium hydroxide to produce a salt and hydrogen.
C It reacts with ammonium salts to produce ammonia.
D It turns red litmus blue.
Answer/Explanation
Ans: A
Question
Three statements about synthetic polymers are listed.
1 Man-made fibres are used for making clothing.
2 Plastics can cause pollution problems both on land and at sea.
3 Plastics which do not rot away are described as non-biodegradable.
Which statements are correct?
A 1 and 2 only B 1 and 3 only C 2 and 3 only D 1, 2 and 3
Answer/Explanation
Ans: D
